Page 452 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 452
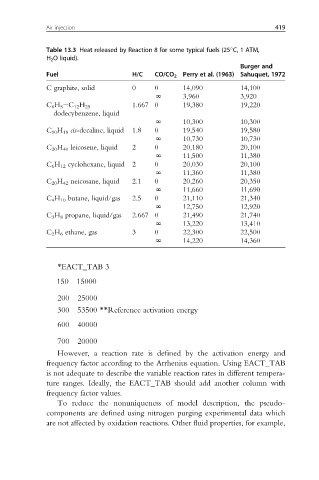
Air injection 419
Table 13.3 Heat released by Reaction 8 for some typical fuels (25 C, 1 ATM,
H 2 O liquid).
Burger and
Fuel H/C CO/CO 2 Perry et al. (1963) Sahuquet, 1972
C graphite, solid 0 0 14,090 14,100
N 3,960 3,920
1.667 0 19,380 19,220
C 6 H 5 eC 12 H 25
dodecybenzene, liquid
N 10,300 10,300
C 10 H 18 cis-decaline, liquid 1.8 0 19,540 19,580
N 10,730 10,730
C 20 H 40 leicosene, liquid 2 0 20,180 20,100
N 11,500 11,380
C 6 H 12 cyclohexane, liquid 2 0 20,030 20,100
N 11,360 11,380
C 20 H 42 neicosane, liquid 2.1 0 20,260 20,350
N 11,660 11,690
C 4 H 10 butane, liquid/gas 2.5 0 21,110 21,340
N 12,750 12,920
C 3 H 8 propane, liquid/gas 2.667 0 21,490 21,740
N 13,220 13,410
C 2 H 6 ethane, gas 3 0 22,300 22,500
N 14,220 14,360
*EACT_TAB 3
150 15000
200 25000
300 53500 **Reference activation energy
600 40000
700 20000
However, a reaction rate is defined by the activation energy and
frequency factor according to the Arrhenius equation. Using EACT_TAB
is not adequate to describe the variable reaction rates in different tempera-
ture ranges. Ideally, the EACT_TAB should add another column with
frequency factor values.
To reduce the nonuniqueness of model description, the pseudo-
components are defined using nitrogen purging experimental data which
are not affected by oxidation reactions. Other fluid properties, for example,