Page 390 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 390
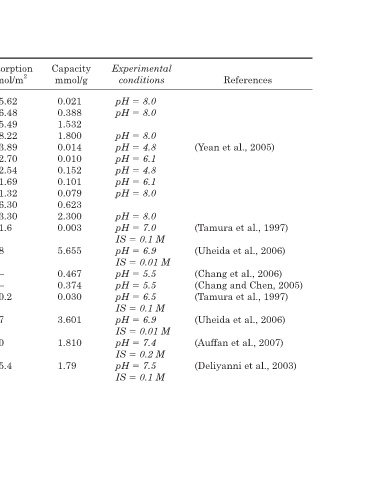
References (Yean et al., 2005) (Tamura et al., 1997) (Uheida et al., 2006) (Chang et al., 2006) (Chang and Chen, 2005) (Tamura et al., 1997) (Uheida et al., 2006) (Auffan et al., 2007) (Deliyanni et al., 2003)
Experimental conditions 8.0 pH 8.0 pH 8.0 pH 4.8 pH 6.1 pH 4.8 pH 6.1 pH 8.0 pH 8.0 pH 7.0 pH 0.1 M IS 6.9 pH 0.01 M IS 5.5 pH 5.5 pH 6.5 pH 0.1 M IS 6.9 pH 0.01 M IS 7.4 pH 0.2 M IS 7.5 pH 0.1 M IS
Capacity mmol/g 0.021 0.388 1.532 1.800 0.014 0.010 0.152 0.101 0.079 0.623 2.300 0.003 5.655 0.467 0.374 0.030 3.601 1.810 1.79
Adsorption mol/m 2 5.62 6.48 15.49 18.22 3.89 2.70 2.54 1.69 1.32 6.30 23.30 1.6 58 – – 0.2 37 10 5.4
Adsorbates As III As III As III As V As V As V Co II Co II Co II Cu II Co II Co II As III,V As V
Adsorption Capacity of Oxide Particles SSA * (m 2 /g) Size (nm) 3.7 300 60 20 98.8 11.72 3.7 300 60 20 98.8 11.72 1.73 1000 97.5 11.3 (86.43) 13.5 (86.43) 13.5 15.9 10000 (114.50) 10 174 6 330 2.6
TABLE 10.1 Adsorbents mineral Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Fe 3 O 4 Chitosan Fe 3 O 4 Chitosan Fe 3 O 4 α-Fe 2 O 3 γ- Fe 2 O 3 γ- Fe 2 O 3 β-FeOOH
374