Page 449 - Handbook of Properties of Textile and Technical Fibres
P. 449
422 Handbook of Properties of Textile and Technical Fibres
• PTT has the best soft handleability of all; PBT is close to PET.
• PTT and PBT can be easily dyed at 100 C; for dyeing of PET higher temperatures (130 C)
are necessary.
The poly(ethylene-2,6-naphthalate) (PEN) fiber containing naphthalene ring are
good candidates for technical applications because they have better thermal stability
and mechanical properties than PET fibers (see Section 13.5). Polyester fibers can
be readily modified and easily textured. It is possible to modify their elasticity, pilling
tendency, dye ability, as well as shrinkage properties (Militký et al., 1991).
The first part of this chapter reviews the manufacturing techniques of standard poly-
ester fibers and their modifications. The basic routes for synthesizing and treatment of
polyesters are discussed.
In the second part, the technologies of spinning, drawing and heat setting, and the
corresponding structural changes are presented.
The third part is devoted to the description of the mechanical behavior and tensile
failure of polyester fibers. At the same time the influence of internal structure on the
mechanical characteristics of polyester fibers is discussed.
In the fourth part, PEN fibers and polyester fibers with PEN as the modification
component are briefly described.
13.2 Chemistry and production of polyester fibers
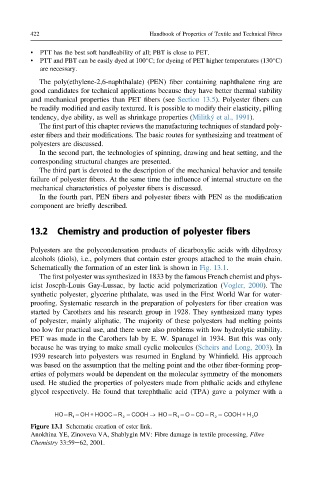
Polyesters are the polycondensation products of dicarboxylic acids with dihydroxy
alcohols (diols), i.e., polymers that contain ester groups attached to the main chain.
Schematically the formation of an ester link is shown in Fig. 13.1.
The first polyester was synthesized in 1833 by the famous French chemist and phys-
icist Joseph-Louis Gay-Lussac, by lactic acid polymerization (Vogler, 2000). The
synthetic polyester, glycerine phthalate, was used in the First World War for water-
proofing. Systematic research in the preparation of polyesters for fiber creation was
started by Carothers and his research group in 1928. They synthesized many types
of polyester, mainly aliphatic. The majority of these polyesters had melting points
too low for practical use, and there were also problems with low hydrolytic stability.
PET was made in the Carothers lab by E. W. Spanagel in 1934. But this was only
because he was trying to make small cyclic molecules (Scheirs and Long, 2003). In
1939 research into polyesters was resumed in England by Whinfield. His approach
was based on the assumption that the melting point and the other fiber-forming prop-
erties of polymers would be dependent on the molecular symmetry of the monomers
used. He studied the properties of polyesters made from phthalic acids and ethylene
glycol respectively. He found that terephthalic acid (TPA) gave a polymer with a
HO– R – OH + HOOC – R – COOH → HO – R – O – CO – R – COOH + H O
1 2 1 2 2
Figure 13.1 Schematic creation of ester link.
Anokhina YE, Zinoveva VA, Shablygin MV: Fibre damage in textile processing, Fibre
Chemistry 33:59e62, 2001.