Page 570 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 570
Hybrid materials based on polymer nanocomposites for environmental applications 523
by absorption of light, the bandgap of the material should be chosen to absorb a large
wavelength range of the visible light.
These considerations lead to the choice of hybrid materials, which are composed of
nanostructured inorganic oxide (nanoparticles, nanowires, or nanorods) embedded in
a polymer matrix. The polymers used in these composites are usually conjugated and
have a low bandgap for extending the light absorption range of metal oxides, which are
generally wide bandgap materials. The most popular photocatalyst is TiO 2 , which is
nontoxic, cheap, and photoreactive. Because of its large bandgap (EG¼3.2 eV), the
oxide absorbs only ultraviolet irradiation of sunlight, which represents <5% of the
solar spectrum. It is possible to modify the bandgap of TiO 2 , especially anatase, by
cation or anion doping to improve optical absorption and consequently photocatalytic
performance [78], but the use of TiO 2 composites can provide similar effects as will be
explained below.
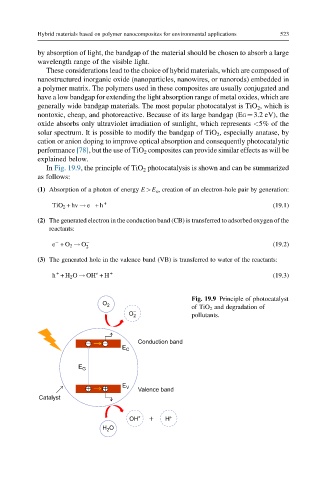
In Fig. 19.9, the principle of TiO 2 photocatalysis is shown and can be summarized
as follows:
(1) Absorption of a photon of energy E>E G , creation of an electron-hole pair by generation:
TiO 2 +hv ! e +h + (19.1)
(2) The generated electron in the conduction band (CB) is transferred to adsorbed oxygen of the
reactants:
e +O 2 ! O 2 (19.2)
(3) The generated hole in the valence band (VB) is transferred to water of the reactants:
+
h +H 2 O ! OH ∗ +H + (19.3)
Fig. 19.9 Principle of photocatalyst
O 2 of TiO 2 and degradation of
O – 2 pollutants.
Conduction band
E C
E G
E
V
Valence band
Catalyst
OH ∗ + H +
H O
2