Page 155 - Algae Anatomy, Biochemistry, and Biotechnology
P. 155
138 Algae: Anatomy, Biochemistry, and Biotechnology
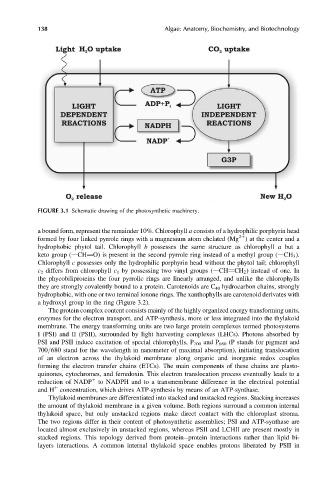
FIGURE 3.1 Schematic drawing of the photosynthetic machinery.
a bound form, represent the remainder 10%. Chlorophyll a consists of a hydrophilic porphyrin head
2þ
formed by four linked pyrrole rings with a magnesium atom chelated (Mg ) at the center and a
hydrophobic phytol tail. Chlorophyll b possesses the same structure as chlorophyll a but a
keto group (22CH55O) is present in the second pyrrole ring instead of a methyl group (22CH 3 ).
Chlorophyll c possesses only the hydrophilic porphyrin head without the phytol tail; chlorophyll
c 2 differs from chlorophyll c 1 by possessing two vinyl groups (22CH55CH 2 ) instead of one. In
the phycobiliproteins the four pyrrolic rings are linearly arranged, and unlike the chlorophylls
they are strongly covalently bound to a protein. Carotenoids are C 40 hydrocarbon chains, strongly
hydrophobic, with one or two terminal ionone rings. The xanthophylls are carotenoid derivates with
a hydroxyl group in the ring (Figure 3.2).
The protein complex content consists mainly of the highly organized energy transforming units,
enzymes for the electron transport, and ATP-synthesis, more or less integrated into the thylakoid
membrane. The energy transforming units are two large protein complexes termed photosystems
I (PSI) and II (PSII), surrounded by light harvesting complexes (LHCs). Photons absorbed by
PSI and PSII induce excitation of special chlorophylls, P 700 and P 680 (P stands for pigment and
700/680 stand for the wavelength in nanometer of maximal absorption), initiating translocation
of an electron across the thylakoid membrane along organic and inorganic redox couples
forming the electron transfer chains (ETCs). The main components of these chains are plasto-
quinones, cytochromes, and ferredoxin. This electron translocation process eventually leads to a
þ
reduction of NADP to NADPH and to a transmembrane difference in the electrical potential
þ
and H concentration, which drives ATP-synthesis by means of an ATP-synthase.
Thylakoid membranes are differentiated into stacked and unstacked regions. Stacking increases
the amount of thylakoid membrane in a given volume. Both regions surround a common internal
thylakoid space, but only unstacked regions make direct contact with the chloroplast stroma.
The two regions differ in their content of photosynthetic assemblies; PSI and ATP-synthase are
located almost exclusively in unstacked regions, whereas PSII and LCHII are present mostly in
stacked regions. This topology derived from protein–protein interactions rather than lipid bi-
layers interactions. A common internal thylakoid space enables protons liberated by PSII in