Page 274 - Materials Chemistry, Second Edition
P. 274
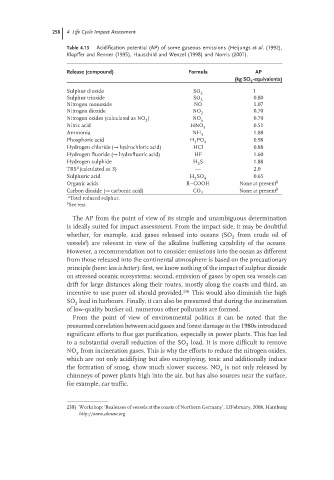
258 4 Life Cycle Impact Assessment
Table 4.13 Acidification potential (AP) of some gaseous emissions (Heijungs et al. (1992),
Kl¨ opffer and Renner (1995), Hauschild and Wenzel (1998) and Norris (2001).
Release (compound) Formula AP
(kg SO -equivalents)
2
Sulphur dioxide SO 2 1
Sulphur trioxide SO 0.80
3
Nitrogen monoxide NO 1.07
Nitrogen dioxide NO 0.70
2
Nitrogen oxides (calculated as NO ) NO x 0.70
2
Nitric acid HNO 0.51
3
Ammonia NH 3 1.88
Phosphoric acid H PO 4 0.98
3
Hydrogen chloride (→ hydrochloric acid) HCl 0.88
Hydrogen fluoride (→ hydrofluoric acid) HF 1.60
Hydrogen sulphide H S 1.88
2
a
TRS (calculated as S) — 2.0
Sulphuric acid H SO 0.65
2 4
Organic acids R–COOH None at present b
Carbon dioxide (→ carbonic acid) CO None at present b
2
a Total reduced sulphur.
b See text.
The AP from the point of view of its simple and unambiguous determination
is ideally suited for impact assessment. From the impact side, it may be doubtful
whether, for example, acid gases released into oceans (SO from crude oil of
2
vessels!) are relevant in view of the alkaline buffering capability of the oceans.
However, a recommendation not to consider emissions into the ocean as different
from those released into the continental atmosphere is based on the precautionary
principle (here: less is better): first, we know nothing of the impact of sulphur dioxide
on stressed oceanic ecosystems; second, emission of gases by open sea vessels can
drift for large distances along their routes, mostly along the coasts and third, an
incentive to use purer oil should provided. 238) This would also diminish the high
SO load in harbours. Finally, it can also be presumed that during the incineration
2
of low-quality bunker oil, numerous other pollutants are formed.
From the point of view of environmental politics it can be noted that the
presumed correlation between acid gases and forest damage in the 1980s introduced
significant efforts to flue gas purification, especially in power plants. This has led
to a substantial overall reduction of the SO load. It is more difficult to remove
2
NO from incineration gases. This is why the efforts to reduce the nitrogen oxides,
x
which are not only acidifying but also eutrophying, toxic and additionally induce
the formation of smog, show much slower success. NO is not only released by
x
chimneys of power plants high into the air, but has also sources near the surface,
for example, car traffic.
238) Workshop: ‘Realeases of vessels at the coasts of Northern Germany’, 12February, 2008, Hamburg
http://www.aknew.org