Page 176 - Materials Chemistry, Second Edition
P. 176
Fungal Treatment of Pharmaceuticals in Effluents 157
MnP, VP, and laccase along with other accessory enzymes. The PhACs in the influ-
ent of WWTPs present a broad variety of compounds with different concentrations.
Different groups of pharmaceuticals, including anti-inflammatory drugs, analgesic
drugs, psychotropic drugs, lipid regulators, antibiotics, β-blockers, estrogens, and
iodinated contrast media can be degraded effectively by WRF (see Table 8.1) with
various reactor configurations.
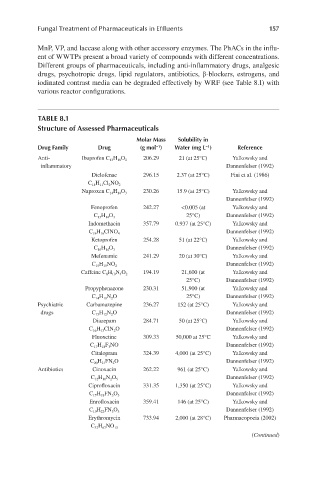
TABLE 8.1
Structure of Assessed Pharmaceuticals
Molar Mass Solubility in
Drug Family Drug (g mol ) Water (mg L ) Reference
−1
−1
Anti- Ibuprofen C 13 H 18 O 2 206.29 21 (at 25°C) Yalkowsky and
inflammatory Dannenfelser (1992)
Diclofenac 296.15 2.37 (at 25°C) Fini et al. (1986)
C 14 H 11 Cl 2 NO 2
230.26 15.9 (at 25°C) Yalkowsky and
Naproxen C 14 H 14 O 3
Dannenfelser (1992)
Fenoprofen 242.27 <0.005 (at Yalkowsky and
25°C) Dannenfelser (1992)
C 15 H 14 O 3
Indomethacin 357.79 0.937 (at 25°C) Yalkowsky and
Dannenfelser (1992)
C 19 H 16 ClNO 4
Ketoprofen 254.28 51 (at 22°C) Yalkowsky and
Dannenfelser (1992)
C 16 H 14 O 3
Mefenamic 241.29 20 (at 30°C) Yalkowsky and
Dannenfelser (1992)
C 15 H 15 NO 2
194.19 21,600 (at Yalkowsky and
Caffeine C 8 H 10 N 4 O 2
25°C) Dannenfelser (1992)
Propyphenazone 230.31 51,900 (at Yalkowsky and
C 14 H 18 N 2 O 25°C) Dannenfelser (1992)
Psychiatric Carbamazepine 236.27 152 (at 25°C) Yalkowsky and
drugs C 15 H 12 N 2 O Dannenfelser (1992)
Diazepam 284.71 50 (at 25°C) Yalkowsky and
C 16 H 13 ClN 2 O Dannenfelser (1992)
Fluoxetine 309.33 50,000 at 25°C Yalkowsky and
C 17 H 18 F 3 NO Dannenfelser (1992)
Citalopram 324.39 4,000 (at 25°C) Yalkowsky and
C 20 H 21 FN 2 O Dannenfelser (1992)
Antibiotics Cinoxacin 262.22 961 (at 25°C) Yalkowsky and
Dannenfelser (1992)
C 12 H 10 N 2 O 5
Ciprofloxacin 331.35 1,350 (at 25°C) Yalkowsky and
Dannenfelser (1992)
C 17 H 18 FN 3 O 3
Enrofloxacin 359.41 146 (at 25°C) Yalkowsky and
Dannenfelser (1992)
C 19 H 22 FN 3 O 3
Erythromycin 733.94 2,000 (at 28°C) Pharmacopoeia (2002)
C 37 H 67 NO 13
(Continued)