Page 113 - Materials Chemistry, Second Edition
P. 113
96 Practical Design Calculations for Groundwater and Soil Remediation
The diffusion coefficient of a chemical in water can be estimated by using
the Wilke–Chang method [8]:
5.06 × 10 −7 T
D = 0.6 (3.26)
0
µ w V
where
D = diffusion coefficient, in cm /s
2
0
T = temperature, in K
μ = viscosity of water, in cP (see Table 3.2)
w
V = molal volume of the solute at its normal boiling point, in cm /g
3
mole
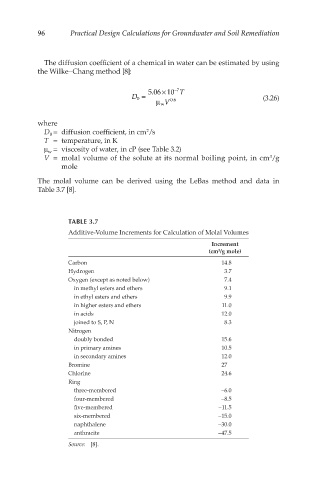
The molal volume can be derived using the LeBas method and data in
Table 3.7 [8].
TABLE 3.7
Additive-Volume Increments for Calculation of Molal Volumes
Increment
(cm /g mole)
3
Carbon 14.8
Hydrogen 3.7
Oxygen (except as noted below) 7.4
in methyl esters and ethers 9.1
in ethyl esters and ethers 9.9
in higher esters and ethers 11.0
in acids 12.0
joined to S, P, N 8.3
Nitrogen
doubly bonded 15.6
in primary amines 10.5
in secondary amines 12.0
Bromine 27
Chlorine 24.6
Ring
three-membered −6.0
four-membered −8.5
five-membered −11.5
six-membered −15.0
naphthalene −30.0
anthracite −47.5
Source: [8].