Page 170 - Materials Chemistry, Second Edition
P. 170
Vadose Zone Soil Remediation 153
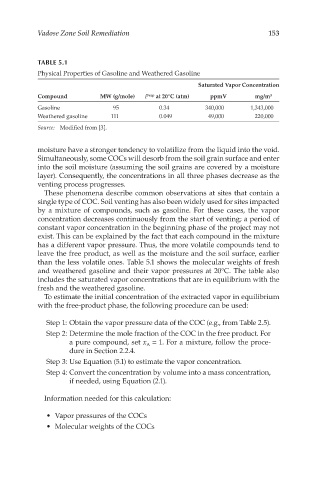
TABLE 5.1
Physical Properties of Gasoline and Weathered Gasoline
Saturated Vapor Concentration
Compound MW (g/mole) P vap at 20°C (atm) ppmV mg/m 3
Gasoline 95 0.34 340,000 1,343,000
Weathered gasoline 111 0.049 49,000 220,000
Source: Modified from [3].
moisture have a stronger tendency to volatilize from the liquid into the void.
Simultaneously, some COCs will desorb from the soil grain surface and enter
into the soil moisture (assuming the soil grains are covered by a moisture
layer). Consequently, the concentrations in all three phases decrease as the
venting process progresses.
These phenomena describe common observations at sites that contain a
single type of COC. Soil venting has also been widely used for sites impacted
by a mixture of compounds, such as gasoline. For these cases, the vapor
concentration decreases continuously from the start of venting; a period of
constant vapor concentration in the beginning phase of the project may not
exist. This can be explained by the fact that each compound in the mixture
has a different vapor pressure. Thus, the more volatile compounds tend to
leave the free product, as well as the moisture and the soil surface, earlier
than the less volatile ones. Table 5.1 shows the molecular weights of fresh
and weathered gasoline and their vapor pressures at 20°C. The table also
includes the saturated vapor concentrations that are in equilibrium with the
fresh and the weathered gasoline.
To estimate the initial concentration of the extracted vapor in equilibrium
with the free-product phase, the following procedure can be used:
Step 1: Obtain the vapor pressure data of the COC (e.g., from Table 2.5).
Step 2: Determine the mole fraction of the COC in the free product. For
a pure compound, set x = 1. For a mixture, follow the proce-
A
dure in Section 2.2.4.
Step 3: Use Equation (5.1) to estimate the vapor concentration.
Step 4: Convert the concentration by volume into a mass concentration,
if needed, using Equation (2.1).
Information needed for this calculation:
• Vapor pressures of the COCs
• Molecular weights of the COCs