Page 167 - A Practical Introduction to Optical Mineralogy
P. 167
THE NON-SILICATES OXIDES
Iron-titanium oxides
Tetrahedrite Cu,o(Zn,Fe ),Sb 4 S 13
Tetrahedrite exhibits extensive chemical substitution and often contains Minerals with chemical compositions of essentially iron, titanium and
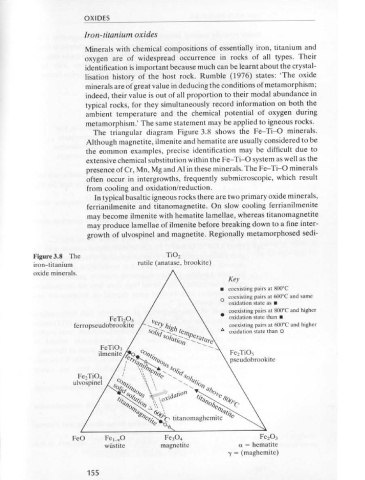
Ag, Hg and As but only rarely Cd, Bi and Pb. The arsenic end member is oxygen are of widespread occurrence in rocks of all types. Their
tennantite Cu, 0 (Zn,Fe) 2 As 4 S 13 • Silver-rich tetrahedrite is known as identjfication is important because much can be learnt about the crystal-
freibergite. Tetrahedrite-tennantites were often formerly called fahlerz. lisation history of the host rock. Rumble {1976) states: 'The oxide
Crystals Tetrahedrite is cubic and occurs as modified tetrahedra. Twinning on the minerals are of great value in deducing the conditions of metamorphism;
axis [ 111 J is often repeated. There are also penetration twins. There is indeed, their value is out of all proportion to their modal abundance in
no cleavage. D = 5.0. typical rocks, for they simultaneously record information on both the
Thin section Tetrahedrites are usually opaque, but iron-free and arsenic-rich var- ambient temperature and the chemical potential of oxygen during
ieties transmit some red light. metamorphism .' The same statement may be api?Iied to igneous rocks.
Polished Tetrahedrite is light grey, sometimes appearing slightly greenish, bluish The triangular diagram Figure 3.8 shows the Fe-Ti-0 minerals.
section or brownish. With R = 31% it is darker than galena but brighter than Although magnetite, ilmenite and hematite are usually considered to be
sphalerite. It is usually isotropic but may be weakly anisotropic. Very the eommon examples, precise identification may be difficult due to
scarce red internal reflections have been reported from tennantite. extensive chemical substitution within the Fe-Ti-0 system as well as the
Tetrahedrite is rarely idiomorphic. It is usually in the form of rounded presence of Cr, Mn, Mg and AI in these minerals. The Fe-Ti-0 minerals
grains or polycrystalline aggregates. It forms myrmekitic intergrowths often occur in intergrowths, frequently submicroscopic, which result
with other sulphides, e.g. galena, chalcopyrite. Zonation of Sb/ As and from cooling and oxidation/reduction.
Fe/Zn is commonly detected on microanalysis but is not visible in In typical basaltic igneous rocks there are two primary oxide minerals,
polished section. Irregular fracturing is common. Inclusions, especially ferrianilmenite and titanomagnetite. On slow cooling ferrianilmenite
of chalcopyrite, are common. VHN = 320-367. may become ilmenite with hematite lamellae, whereas titanomagnetite
Occurrence Tetrahedrite commonly occurs associated with galena in lead + zinc may produce lamellae of ilmenite before breaking down to a fine inter-
deposits, although it is inexplicably abundant in some and absent in growth of ulvospinel and magnetite. Regionally metamorphosed sedi-
others. Tennantite is common in porphyry copper mineralisation.
Distinguishing Compared with tetrahedrites, sphalerite is darker, harder, has a good
features cleavage and usually shows internal reflections. Many complex sul- Figure 3.8 The Ti0 2
iron-titanium rutile (anatase, brookite)
phides (sulphosalts) are simlar at first glance to tetrahedrite, but most of
oxide minerals.
these are anisotropic. Key
Notes The various chemical varieties of tetrahedrite cannot be identified with • coexisting pairs at 800°C
any certainty in polished section without resorting to microanalysis. 0 coexisting pairs at 600°C and same
oxidation state as •
coexisting pairs at 800°C and higher
FeTizOs • oxidation state than •
ferropseudobrookite coexisting pairs at 600°C and higher
3.4 Oxides t; oxidation state than 0
Oxides are minerals that contain one or more metals and oxygen; quartz Fe 2 Ti0s
pseudobrookite
Si0 2 is usually excluded from the group. The reader is referred to
Rumble {1976) for a review of the oxides. As most silicates in igneous
Fe 2 Ti04
and metamorphic rocks consist essentially of silica plus metal oxides, ulvospinel
free oxides can be considered as forming if metal oxides are present
surplus to the needs of silicates. Alternatively, they may form if rocks are
silica deficient, as is usually the case when periclase MgO and corundum
Al 2 0 3 are found, or they may form if the metal is 'inappropriate' for a
silicate structure, e.g. cassiterite Sn0 2 • The two following groups, the
iron-titanium oxides and the spinels, overlap to a certain degree but will FeO Fe,_.o Fez OJ
be outlined briefly because they contain the most common oxides. Note wiistite a= hematite
that all the oxides are listed alphabetically. -y = (maghemite)
154 155