Page 19 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 19
Introduction to Physical Chemistry 3
species. e.g. OH-. An acid combines with a base to form a salt and
water:
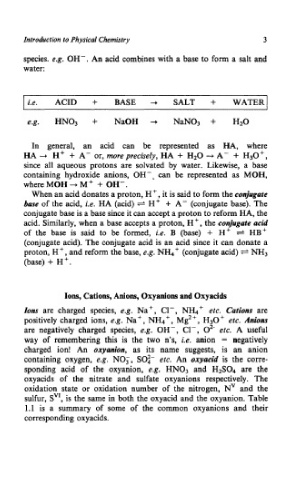
I i.e. ACID + BASE -+ SALT + WATER^
e.g. HN03 + NaOH -+ NaN03 + H20
In general, an acid can be represented as HA, where
HA -+ H+ + A- or, more precisely, HA + H20 -+ A- + H30+,
since all aqueous protons are solvated by water. Likewise, a base
containing hydroxide anions, OH-, can be represented as MOH,
where MOH ---$ M+ + OH-.
When an acid donates a proton, H+ , it is said to form the conjugate
base of the acid, i.e. HA (acid) * H+ + A- (conjugate base). The
conjugate base is a base since it can accept a proton to reform HA, the
acid. Similarly, when a base accepts a proton, H+ , the conjugate acid
of the base is said to be formed, i.e. B (base) + H+ HB+
(conjugate acid). The conjugate acid is an acid since it can donate a
proton, H+ , and reform the base, e.g. NH4+ (conjugate acid) --+ NH3
(base) + H+.
Ions, Cations, Anions, Oxyanions and Oxyacids
Ions are charged species, e.g. Na+, Cl-, NH4+ etc. Cations are
positively charged ions, e.g. Na+, NH4+, Mg2+, H30+ etc. Anions
are negatively charged species, e.g. OH-, C1-, 02- etc. A useful
way of remembering this is the two n’s, i.e. anion = negatively
charged ion! An oxymion, as its name suggests, is an anion
containing oxygen, e.g. NO,, SO:- etc. An oxyacid is the corre-
sponding acid of the oxyanion, e.g. HN03 and H2SO4 are the
oxyacids of the nitrate and sulfate oxyanions respectively. The
oxidation state or oxidation number of the nitrogen, Nv and the
sulfur, Svl, is the same in both the oxyacid and the oxyanion. Table
1.1 is a summary of some of the common oxyanions and their
corresponding oxyacids.