Page 48 - A Working Method Approach For Introductory Physical Chemistry Calculations
P. 48
32 Chapter 3
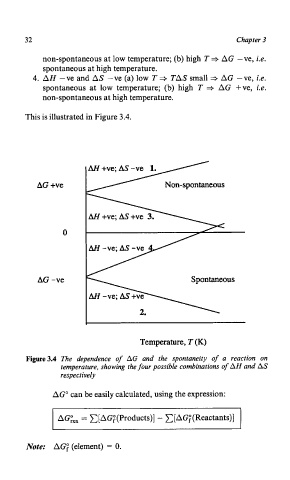
non-spontaneous at low temperature; (b) high T 3 AG -ve, i.e.
spontaneous at high temperature.
4. AH -ve and AS -ve (a) low T TAS small + AG -ve, i.e.
spontaneous at low temperature; (b) high T + AG +ve, i.e.
non-spontaneous at high temperature.
This is illustrated in Figure 3.4.
AG +ve Non-spontaneous
0
AG -ve Spontaneous
Temperature, T (K)
Figure3.4 The dependence of AG and the spontaneity of a reaction on
temperature, showing the four possible combinations of AH and AS
respectively
AGO can be easily calculated, using the expression:
I AGK, = C[AG;(Products)] - x[AG;(Reactants)] I
Note: AG; (element) = 0.