Page 67 - A Working Method Approach For Introductory Physical Chemistry Calculations
P. 67
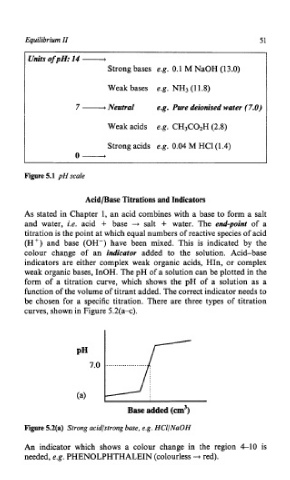
Units ofpH: 14 -
Equilibrium II 51
Strong bases e.g. 0.1 M NaOH (13.0)
Weak bases e.g. NH3 (1 1.8)
7 - Neutral e.g. Pure deionised water (7.0)
Weak acids e.g. CH3C02H (2.8)
Strong acids e.g. 0.04 M HCl(l.4)
0-
Figure 5.1 pH scale
Acid/Base Titrations and Indicators
As stated in Chapter 1, an acid combines with a base to form a salt
and water, i.e. acid + base -+ salt + water. The end-point of a
titration is the point at which equal numbers of reactive species of acid
(H+) and base (OH-) have been mixed. This is indicated by the
colour chavge of an indicator added to the solution. Acid-base
indicators are either complex weak organic acids, HIn, or complex
weak organic bases, InOH. The pH of a solution can be plotted in the
form of a titration curve, which shows the pH of a solution as a
function of the volume of titrant added. The correct indicator needs to
be chosen for a specific titration. There are three types of titration
curves, shown in Figure 5.2(a-c).
-
7.0 .__ _. _._.. .._ ._ ___.
Base added (cm3)
Figure 5.2(a) Strong acidlstrong base, e.g. HCllNaOH
An indicator which shows a colour change in the region 4-10 is
needed, e.g. PHENOLPHTHALEIN (colourless + red).