Page 185 - Adsorbents - fundamentals and applications
P. 185
170 ZEOLITES AND MOLECULAR SIEVES
role of the templating agent in organizing and shaping structural voids during
crystallization. The template in most cases remains entrapped in these voids and
is removed by calcination. Some of the AlPO 4 types, such as AlPO 4 -5, can be
synthesized with many different templates. The AlPO 4 s are formed by tetrahe-
drally coordinated AlO 4 and PO 4 , and have no need for charge-balancing cations.
A large number of silicoaluminophosphate (SAPO 4 ) analogs have also been syn-
thesized. The syntheses and characterization of a high amount of SAPO 4 and
SAPO 4 have been reviewed by Szostak (1998), Ernst (1998), and Hartmann and
Kevan (1999). The SAPO 4 analogs are formed by silicon, aluminum, phospho-
rous, and oxygen atoms in tetrahedral coordination, with uniform pore channels
in molecular dimension (Lok et al., 1984; Lok et al., 1988). SAPO 4 ’s have a
framework with a net charge that varies depending on how the silicon is sub-
stituted into the aluminophosphate. That is, if silicon substitutes for aluminum,
phosphorous, or both, the resulting net charge will be, respectively, +1, −1, or 0
(Djieugoue, et al., 1999). Usually the second and third substitutions occur during
the crystallization process.
The rich variety of pore structures, both cavities and channels, as well as
the cation sites that can be exchanged in the SAPO 4 analogs, offer promising
opportunities for their use as new sorbents for separations. An example for such
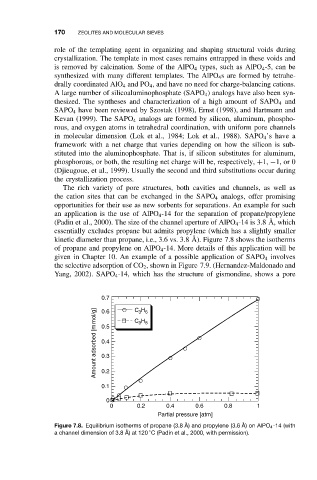
an application is the use of AlPO 4 -14 for the separation of propane/propylene
(Padin et al., 2000). The size of the channel aperture of AlPO 4 -14 is 3.8 ˚ A, which
essentially excludes propane but admits propylene (which has a slightly smaller
kinetic diameter than propane, i.e., 3.6 vs. 3.8 ˚ A). Figure 7.8 shows the isotherms
of propane and propylene on AlPO 4 -14. More details of this application will be
given in Chapter 10. An example of a possible application of SAPO 4 involves
the selective adsorption of CO 2 , shown in Figure 7.9. (Hernandez-Maldonado and
Yang, 2002). SAPO 4 -14, which has the structure of gismondine, shows a pore
0.7 C H
Amount adsorbed [m mol/g] 0.5 C H
0.6
3 6
3 8
0.4
0.3
0.2
0.1
0
0 0.2 0.4 0.6 0.8 1
Partial pressure [atm]
Figure 7.8. Equilibrium isotherms of propane (3.8 ˚ A) and propylene (3.6 ˚ A) on AlPO 4 -14 (with
◦
a channel dimension of 3.8 ˚ A) at 120 C (Padin et al., 2000, with permission).