Page 29 - Adsorbents - fundamentals and applications
P. 29
14 FUNDAMENTAL FACTORS FOR DESIGNING ADSORBENT
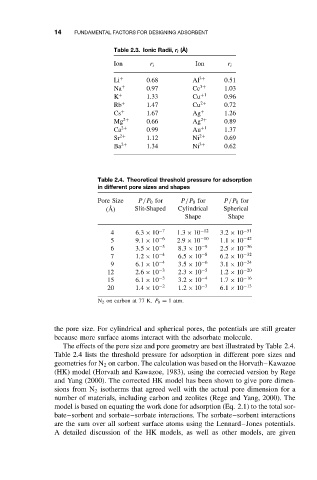
Table 2.3. Ionic Radii, r i ( ˚ A)
Ion r i Ion r i
Li + 0.68 Al 3+ 0.51
Na + 0.97 Ce 3+ 1.03
K + 1.33 Cu +1 0.96
Rb + 1.47 Cu 2+ 0.72
Cs + 1.67 Ag + 1.26
Mg 2+ 0.66 Ag 2+ 0.89
Ca 2+ 0.99 Au +1 1.37
Sr 2+ 1.12 Ni 2+ 0.69
Ba 2+ 1.34 Ni 3+ 0.62
Table 2.4. Theoretical threshold pressure for adsorption
in different pore sizes and shapes
Pore Size P/P 0 for P/P 0 for P/P 0 for
( ˚ A) Slit-Shaped Cylindrical Spherical
Shape Shape
4 6.3 × 10 −7 1.3 × 10 −12 3.2 × 10 −51
5 9.1 × 10 −6 2.9 × 10 −10 1.1 × 10 −42
6 3.5 × 10 −5 8.3 × 10 −9 2.5 × 10 −36
7 1.2 × 10 −4 6.5 × 10 −8 6.2 × 10 −32
9 6.1 × 10 −4 3.5 × 10 −6 3.1 × 10 −24
12 2.6 × 10 −3 2.3 × 10 −5 1.2 × 10 −20
15 6.1 × 10 −3 3.2 × 10 −4 1.7 × 10 −16
20 1.4 × 10 −2 1.2 × 10 −3 6.1 × 10 −13
N 2 on carbon at 77 K. P 0 = 1atm.
the pore size. For cylindrical and spherical pores, the potentials are still greater
because more surface atoms interact with the adsorbate molecule.
The effects of the pore size and pore geometry are best illustrated by Table 2.4.
Table 2.4 lists the threshold pressure for adsorption in different pore sizes and
geometries for N 2 on carbon. The calculation was based on the Horvath–Kawazoe
(HK) model (Horvath and Kawazoe, 1983), using the corrected version by Rege
and Yang (2000). The corrected HK model has been shown to give pore dimen-
sions from N 2 isotherms that agreed well with the actual pore dimension for a
number of materials, including carbon and zeolites (Rege and Yang, 2000). The
model is based on equating the work done for adsorption (Eq. 2.1) to the total sor-
bate–sorbent and sorbate–sorbate interactions. The sorbate–sorbent interactions
are the sum over all sorbent surface atoms using the Lennard–Jones potentials.
A detailed discussion of the HK models, as well as other models, are given