Page 40 - Adsorbents - fundamentals and applications
P. 40
EQUILIBRIUM ISOTHERMS AND DIFFUSION 25
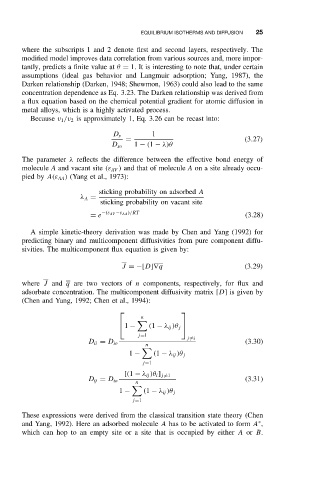
where the subscripts 1 and 2 denote first and second layers, respectively. The
modified model improves data correlation from various sources and, more impor-
tantly, predicts a finite value at θ = 1. It is interesting to note that, under certain
assumptions (ideal gas behavior and Langmuir adsorption; Yang, 1987), the
Darken relationship (Darken, 1948; Shewmon, 1963) could also lead to the same
concentration dependence as Eq. 3.23. The Darken relationship was derived from
a flux equation based on the chemical potential gradient for atomic diffusion in
metal alloys, which is a highly activated process.
Because v 1 /v 2 is approximately 1, Eq. 3.26 can be recast into:
1
D s
= (3.27)
D so 1 − (1 − λ)
The parameter λ reflects the difference between the effective bond energy of
molecule A and vacant site (ε AV ) and that of molecule A on a site already occu-
pied by A(ε AA ) (Yang et al., 1973):
sticking probability on adsorbed A
λ A =
sticking probability on vacant site
= e −(ε AV −ε AA )/RT (3.28)
A simple kinetic-theory derivation was made by Chen and Yang (1992) for
predicting binary and multicomponent diffusivities from pure component diffu-
sivities. The multicomponent flux equation is given by:
J =−[D]∇q (3.29)
where J and q are two vectors of n components, respectively, for flux and
adsorbate concentration. The multicomponent diffusivity matrix [D]isgiven by
(Chen and Yang, 1992; Chen et al., 1994):
n
1 − (1 − λ ij )θ j
j=1
j =i
D ii = D io (3.30)
n
1 − (1 − λ ij )θ j
j=1
[(1 − λ ij )θ i ] j =1
D ij = D io (3.31)
n
1 − (1 − λ ij )θ j
j=1
These expressions were derived from the classical transition state theory (Chen
∗
and Yang, 1992). Here an adsorbed molecule A has to be activated to form A ,
which can hop to an empty site or a site that is occupied by either A or B.