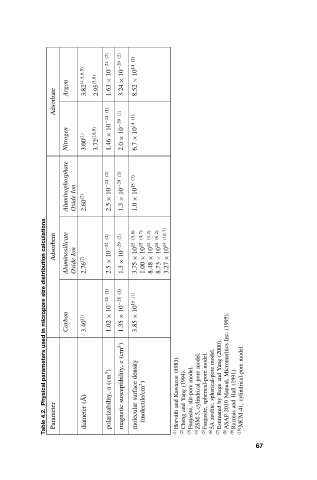
Page 82 - Adsorbents - fundamentals and applications
P. 82
(2) 10 −24 (2) 10 −29 (2) 10 14
Adsorbate Argon 3.82 (4,5,6,9) 2.95 (3,8) (1) × 1.63 × 3.24 × 8.52
10 −24 (1) 10 −29 (1) 10 14
Nitrogen 3.00 (1) 3.72 (10,9) × 1.46 × 2.0 × 6.7
Aluminophosphate Ion Oxide 2.60 (2) (2) 10 −24 × 2.5 (2) 10 −29 × 1.3 (7) 10 15 × 1.0
calculations Adsorbent Ion (2) 10 −24 (2) 10 −29 (3,8) 10 15 (4,7) 10 15 (5,2) 10 14 (6,2) 10 14 (10,7) 10 14
distribution Aluminosilicate Oxide 2.76 (2) × 2.5 × 1.3 × 3.75 × 1.00 × 8.48 × 8.73 × 3.27
size (1) (1) (1)
micropore Carbon 10 −24 × 10 −28 × 10 15 × (1995).
in 3.40 (1) 1.02 1.35 3.85 Inc.
used (cm 3 ) (2000). model.
parameters (cm 3 ) c density (1983). (1994). model. model. model. model. Yang and Micromeritics (1991).
Physical A) ( ˚ a susceptibility, surface (molecule/cm 2 ) Kawazoe and Yang slit-pore cylindrical-pore spherical-pore spherical-pore Rege by Manual, Hall cylindrical-pore
4.2. Parameter diameter polarizability, magnetic molecular ath and zeolite, (7) Estimated 2010 and (10) MCM-41,
Table (1) Horv´ (2) Cheng (3) Faujasite, (4) ZSM-5, (5) Faujasite, (6) 5A (8) ASAP (9) Razmus
67