Page 30 - Adsorptionbypowders & poroussolids muyace
P. 30
ADSORPTIaN BY POWDERSAND POROUS SOWDS
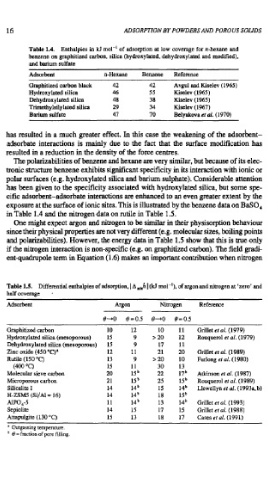
Table 1.4. Enthalpies in Id mol-' of adsorption at low coverage for n-hexane and
benzene on graphitized carbon, silica (hydroxylated, dehydroxylated and modifled),
and barium sulfate
Adsorbent n-Hexane Benzene Reference
Graphitized carbon black 42 42 Avgul and Kiselev (1965)
Hydroxylated silica 46 55 Kiselev (1%5)
Dehydroxylated silica 48 38 Kiselev (1965)
Trimethylsilylated silica 29 34 Kiselev (1967)
Barium sulfate 47 70 Belyakova et al. (1970)
has resulted in a much greater effect. In this case the weakening of the adsorbent-
adsorbate interactions is mainly due to the fact that the surface modification has
resulted in a reduction in the density of the force centres.
The polarizabilities of benzene and hexane are very similar, but because of its elec-
tronic structure benzene exhibits significant specificity in its interaction with ionic or
polar surfaces (e.g. hydroxylated silica and barium sulphate). Considerable attention
has been given to the specificity associated with hydroxylated silica, but some spe-
cific adsorbent-adsorbate interactions are enhanced to an even greater extent by the
exposure at the surface of ionic sites. This is illustrated by the benzene data on BaSO,
in Table 1.4 and the nitrogen data on xutile in Table 1.5.
One might expect argon and nitrogen to be similar in their physisoxption behaviour
since their physical properties are not very different (e.g. molecular sizes, boiling points
and polarizabilities). However, the energy data in Table 1.5 show that this is aue only
if the nitrogen interaction is non-specific (e.g. on graphitized carbon). The field gradi-
ent-quadmpole term in Equation (1.6) makes an important contribution when nitrogen
Table 1s. Differential enthalpies of adsorption, I A d,h I (kJ mol-'), of argon and Ritrogen at 'zero' and
half coverage ,
Adsorbent Argon Nitrogen Reference
Graphitized carbon 10 12 10 11 Grillet ef 01. (1979)
Hydroxylated silica (mesoporous) 15 9 > 20 12 Rouqueml er al. (1979)
Dehydroxylated silica (mesoporous) 15 9 17 11
Zinc oxide (450 "C)' 12 11 2 1 20 Grillet ef of. (1 989)
Rutile (150 "C) 13 9 > 20 10 Furlong et al. (1980)
(400 "C) 15 11 30 13
Molecular sieve carbon 20 15~ 22 17b Atkinson el al. (1987)
Microporous carbon 2 1 15 25 lSb Rouquerol et al. (1989)
Silicalite I 14 14b 15 14b Llewellyn ef al. (1993a, b)
H-ZSMS (Si/AI = 16) 14 14b 18 lSb
AP0,-5 11 14~ 13 14~ Grilleteral.(1993)
Sepiolite 14 15 17 15 Grillet er 01. (1988)
Attapulgite (130 "C) 15 13 18 17 Cases et al. (1991)
* Outgassing temperature.
' 8 =fraction of pore filling.