Page 120 - Adsorption by Powders and Porous Solids
P. 120
,-wTER 4. INTERPRETATION OF PHYSISORPTION ISOTHERMS 105
I
I
LIQUID + SOLID
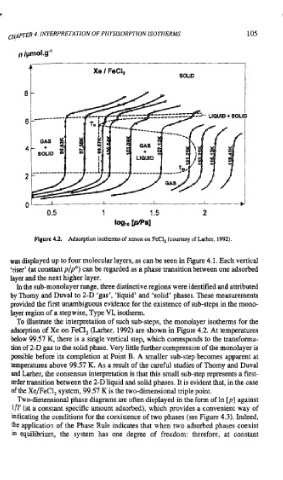
Figure 4.2. Adsorption isotherms of xenon on FeC1, (courtesy of Larher, 1992).
was displayed up to four molecular layers, as can be seen in Figure 4.1. Each vertical
'riser' (at constantp/po) can be regarded as a phase transition between one adsorbed
layer and the next higher layer.
In the sub-monolayer range, three distinctive regions were identified and attributed
by Thomy and Duval to 2-D 'gas', 'liquid' and 'solid' phases. These measurements
provided the first unambiguous evidence for the existence of sub-steps in the mono-
layer region of a stepwise, Type VI, isotherm.
To illustrate the interpretation of such sub-steps, the monolayer isotherms for the
adsorption of Xe on FeC1, (Larher, 1992) are shown in Figure 4.2. At temperatures
below 99.57 K, there is a single vertical step, which corresponds to the transforma-
tion of 2-D gas to the solid phase. Very little further compression of the monolayer is
possible before its completion at Point B. A smaller sub-step becomes apparent at
temperatures above 99.57 K. As a result of the careful studies of Thomy and Duval
and Larher, the consensus interpretation is that this small sub-step represents a first-
order transition between the 2-D liquid and solid phases. It is evident that, in the case
of the Xe/FeCl, system, 99.57 K is the two-dimensional triple point.
Two-dimensional phase diagrams are often displayed in the form of In [p] against
1/T (at a constant specific amount adsorbed), which provides a convenient way of
indicating the conditions for the coexistence of two phases (see Figure 4.3). Indeed,
the application of the Phase Rule indicates that when two adsorbed phases coexist
in equilibrium, the system has one degree of freedom: therefore, at constant