Page 15 - Adsorption by Powders and Porous Solids
P. 15
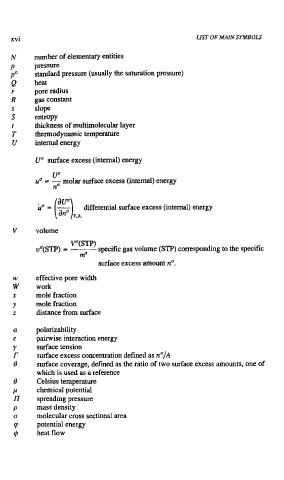
LIST OF MAIN SYMBOLS
number of elementary entities
pressure
standard pressure (usually the saturation pressure)
hear
pore radius
gas constant
slope
entropy
thickness of multimolecular layer
thermodynamic temperature
internal energy
U" surface excess (internal) energy
U"
ua = - molar surface excess (internal) energy
no
differential surface excess (internal) energy
volume
V"(STp)
specific
vU(STP) = - gas volume (STP) corresponding to the specific
ma
surface excess amount nu.
effective pore width
work
mole fraction
mole fraction
distance from surface
polarizability
pairwise interaction energy
surface tension
surface excess concentration defined as n'/~
surface coverage, defined as the ratio of two surface excess amounts, one of
which is used as a reference
Celsius temperature
chemical potential
spreading pressure
mass density
molecular cross sectional area
potential energy
heat flow