Page 156 - Adsorption by Powders and Porous Solids
P. 156
142 ADSORPTION BY POWDERS AND POROUS SOL^^^
5.3.1. Quantitative expression of the amounts adsorbed from a binary solution
Scope and limitations of the normal surface excess amounts
As for adsorption from the gas phase, one must be able to. express
and quantitatively the observed adsorption phenomenon without reference to an
Y
prior knowledge concerning the structure of the adsorbed layer. For this reason, the
concept of the Gibbs dividing surface (GDS) and the associated surface excess
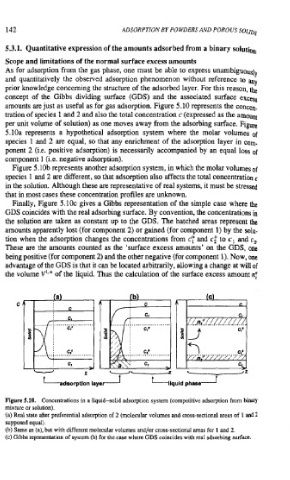
amounts are just as useful as for gas adsorption. Figure 5.10 represents the conwn.
tration of species 1 and 2 and also the total concentration c (expressed as the amom
per unit volume of solution) as one moves away from the adsorbing surface. Figure
5.10a represents a hypothetical adsorption system where the molar volumes of
species 1 and 2 are equal, so that any enrichment of the adsorption layer in corn.
ponent 2 (i.e. positive adsorption) is necessarily accompanied by an equal loss of
component 1 (i.e. negative adsorption).
Figure 5.10b represents another adsorption system, in which the molar volumes of
species 1 and 2 are different, so that adsorption also affects the total concentration
in the solution. Although these are representative of real systems, it must be stressed
that in most cases these concentration profiles are unknown.
Finally, Figure 5.10~ gives a Gibbs representation of the simple case where the
GDS coincides with the real adsorbing surface. By convention, the concentrations in
the solution are taken as constant up to the GDS. The hatched areas represent the
amounts apparently lost (for component 2) or gained (for component 1) by the solu-
tion when the adsorption changes the concentrations from cy and c," to c, and c,.
These are the amounts counted as the 'surface excess amounts' on the GDS, one
being positive (for component 2) and the other negative (for component 1). Now, one
advantage of the GDS is that it can be located arbitrarily, allowing a change at will of
the volume V'*' of the liquid. Thus the calculation of the surface excess amount nj
Figure 5.10. Concentrations in a liquid-solid adsorption system (competitive adsorption from binary
mixture or solution).
(a) Real state aiier preferential adsorption of 2 (molecular volumes and cross-sectional areas of 1 and 2
supposed equal).
(b) Same as (a), but with different molecular volumes and/or cross-sectional areas for 1 and 2.
(c) Gibbs representation of system (b) for the case where GDS coincides with real adsorbing surface.