Page 220 - Adsorption by Powders and Porous Solids
P. 220
ADSOR?TION BY POWDERS AND POROUS souD
condensation occurs in an open-ended cylinder. Since a cylindrical meniscus is' .I
unstable, a spontaneous change leads to the development of an unduloid. In the !
stage, the pore becomes blocked by the formation of a biconcave lens of liquid. & !
evaporation process proceeds in a thermodynamically reversible manner, how
ever, '
and in accordance with the Kelvin equation, the relative pressure is now dependent
on the radius of curvature of the hemispherical menisci. Thus, in the case of thh
simple system, it is the location of the desorption branch which should be used forthe
calculation of r,.
A different approach was adopted by Saam and Cole (1973, who attempted to
explain the hysteresis exhibited by a cylindrical pore in terms of the regimes of
stability, metastability and instability of the multilayer film. The applicability of the
Saam-Cole theory has been explored in some detail by Findenegg et al. (1994),
Lewandowski et al. (1991) and Michalski et al. (1991).
According to the Saam-Cole theory, there are two opposing effects which govern
the range of metastability of a multilayer film in a cylindrical mesopore. Thus, long-
range adsorption forces help to stabilize the film, while capillary forces are respmsi-
ble for the condensation of the liquid. At a critical film thickness, t,, the curved film
becomes unstable and condensation occurs. Evaporation of the hquid condensate
requires a lower p/pO and now the residual film thickness, is t,. We may therefon
regard the difference (t, - t,) as the metastability thickness range of the multilayrn
film.
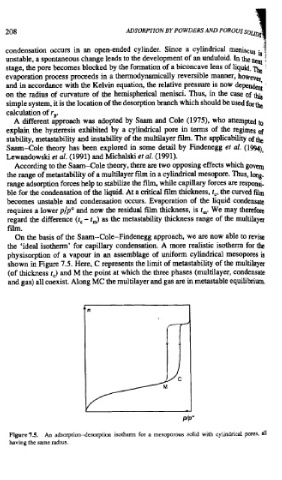
On the basis of the Saam-Cole-Findenegg approach, we are now able to revise
the 'ideal isotherm' for capillary condensation. A more realistic isotherm for the
physisorption of a vapour in an assemblage of uniform cylindrical mesopores is
shown in Figure 7.5. Here, C represents the limit of metastability of the multilayer
(of thickness t,) and M the point at which the three phases (multilayer, condensate
and gas) all coexist. Along MC the multilayer and gas are in metastable equilibrium.
Figure 75. An adsorption-desorption isotherm for a mesoporous solid with cylindrical pores,
having the same radlus.