Page 231 - Adsorption by Powders and Porous Solids
P. 231
220 ADSORPTION BY POWDERS AND POROUS SOUDS
(e) the adsorptive molecular size and shape;
(f) the operational temperature.
In addition, we should keep in mind that the micropore filling capacity 'is dependent
on both the available pore volume and the packing of the adsorbed molecules.
In this chapter, we introduce the currently most popular adsorption methods used
for micropore size analysis: our aim is to outline in general terms the relative merits
and limitations of these procedures. Their application is discussed more fully in later
chapters in relation to the characterization of particular adsorbents.
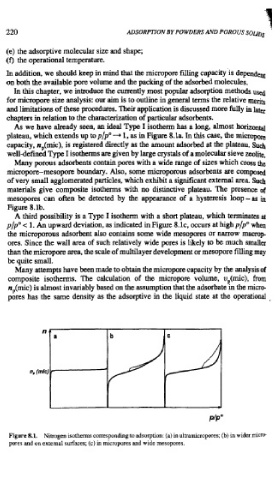
As we have already seen, an ideal Type I isotherm has a long, almost horizontal
plateau, which extends up to plpO 4 1, as in Figure 8. la. In this case, the micropre
capacity, n,(mic), is registered directly as the amount adsorbed at the plateau. Such
well-defined Type I isotherms are given by large crystals of a molecular sieve zeolite,
Many porous adsorbents contain pores with a wide range of sizes which cross the
micropore-mesopore boundary. Also, some microporous adsorbents are composed
of very small agglomerated particles, which exhibit a significant extemal area. Such
materials give composite isotherms with no distinctive plateau. The presence of
mesopores can often be detected by the appearance of a hysteresis loop-as in
Figure 8.lb.
A third possibility is a Type I isotherm with a short plateau, which terminates at
p/pO c 1. An upward deviation, as indicated in Figure 8.lc, occurs at high p/pO when
the microporous adsorbent also contains some wide mesopores or narrow macrop-
ores. Since the wall area of such relatively wide pores is likely to be much smaller
than the micropore area, the scale of multilayer development or mesopore filling may
be quite small.
Many attempts have been made to obtain the micropore capacity by the analysis of
composite isotherms. The calculation of the micropore volume, v,(mic), from
n,(mic) is almost invariably based on the assumption that the adsorbate in the micro-
pores has the same density as the adsorptive in the liquid state at the operational .
"ta c I
Figure 8.1. Nitrogen isotherms corresponding to adsorption: (a) in ultramicropores; (b) in wider micro-
pores and on extemal surfaces; (c) in micropores and wide mesopores.