Page 272 - Adsorption by Powders and Porous Solids
P. 272
ADSORPTlON BY POWDERS AND POROUS SOLlDs
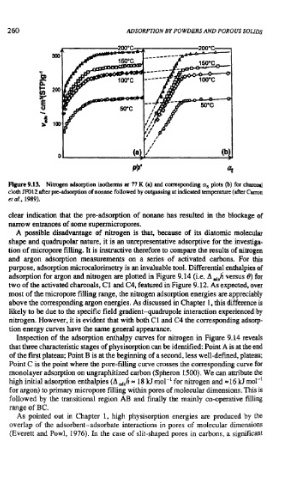
Figure 9.13. Nitrogen adsorption isotherms ar 77 K (a) and corresponding a, plots (b) for chmd
cloth m12 after pre-adsorption of nonane followed by outgassing at indicated temperntun (after Carrot
er a!.. 1989).
clear indication that the pre-adsorption of nonane has resulted in the blockage of
narrow entrances of some supemicropores.
A possible disadvantage of nitrogen is that, because of its diatomic molecular
shape and quadrupolar nature, it is an unrepresentative adsorptive for the investiga-
tion of micropore filling. It is instructive therefore to compare the results of nitrogen
and argon adsorption measurements on a series of activated carbons. For this
purpose, adsorption microcalorimetry is an invaluable tool. Differential enthalpies of
adsorption for argon and nitrogen are plotted in Figure 9.14 (i.e. A ,A versus 8) for
two of the activated charcoals, C1 and C4, featured in Figure 9.12. As expected, over
most of the micropore filling range, the nitrogen adsorption energies are appreciably
above the corresponding argon energies. As discussed in Chapter 1, this difference is
likely to be due to the specific field gradient-quadrupole interaction experienced by
nitrogen. However, it is evident that with both C1 and C4 the corresponding adsorp-
tion energy curves have the same general appearance.
Inspection of the adsorption enthalpy curves for nitrogen in Figure 9.14 reveals
that three characteristic stages of physisorption can be identified: Point A is at the end
of the first plateau; Point B is at the beginning of a second, less well-defined, plateau;
Point C is the point where the pore-filling cwe crosses the corresponding curve for
monolayer adsorption on ungraphitized carbon (Spheron 1500). We can attribute the
high initial adsorption enthalpies (A ,,A = 18 kJ mol-' for nitrogen and =16 kJmol-'
for argon) to primary micropore filling within pores of molecular dimensions. This is
followed by the transitional region AB and finally the mainly co-operative filling
range of BC.
As pointed out in Chapter 1, high physisorption energies are produced by the
overlap of the adsorbent-adsorbate interactions in pores of molecular dimensions
(Everett and Powl, 1976). In the case of slit-shaped pores in carbons, a significant