Page 79 - Adsorption by Powders and Porous Solids
P. 79
ADSORPTION BY POWDERS AND POROUS SOLIDS
adsorptive
external
thermostat
temperature
controlled shield
platinum
resistance
thermometer
adsotbent differential thermocouple
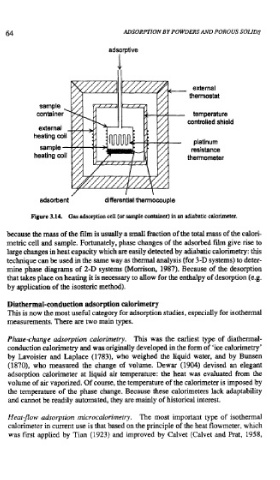
Figure 3.14. Gas adsorption cell (or sample container) in an adiabatic calorimeter.
because the mass of the film is usually a small fraction of the total mass of the calori-
metric cell and sample. Fortunately, phase changes of the adsorbed film give rise to
large changes in heat capacity which are easily detected by adiabatic calorimetry: this
technique can be used in the same way as thermal analysis (for 3-D systems) to deter-
mine phase diagrams of 2-D systems (Momson, 1987). Because of the desorption
that takes place on heating it is necessary to allow for the enthalpy of desorption (e.g.
by application of the isosteric method).
Diathermal-conduction adsorption calorimetry
This is now the most useful category for adsorption studies, especially for isothermal
measurements. There are two main types.
Phase-change adsorption calorimetry. This was the earliest type of diathermal-
conduction calorimetry and was originally developed in the form of 'ice calorimetry'
by Lavoisier and Laplace (1783), who weighed the liquid water, and by Bunsen
(1870), who measured the change of volume. Dewar (1904) devised an elegant
adsorption calorimeter at liquid air temperature: the heat was evaluated from the
volume of air vaporized. Of course, the temperature of the calorimeter is imposed by
the temperature of the phase change. Because these calorimeters lack adaptability
and cannot be readily automated, they are mainly of historical interest.
Heat--ow adsorption microcalorimetry. The most important type of isothermal
calorimeter in current use is that based on the principle of the heat flowmeter, which
was first applied by Tian (1923) and improved by Calvet (Calvet and Prat, 1958,