Page 258 - Adsorption Technology & Design, Elsevier (1998)
P. 258
Selected adsorption processes 233
where t is the time allowed for adsorption in the cycle (equal to desorption)
and k is the mass transfer coefficient for adsorption and desorption. Figure
7.21 illustrates how this function behaves with increasing value of t for
different values of k. It is apparent that, on the one hand, for an adsorption
without mass transfer limitation (k = ~) the net rate of adsorption increases
to infinity as t approaches zero. On the other hand, there is a limiting value of
k/4 as t approaches zero for finite values of k. Furthermore, when t
approaches zero the cycle inefficiency, 11(= 2tR/q*) also becomes zero.
The conclusion to be drawn from this argument is that rapid cycling between
the adsorption pressure pA and the desorption pressure pD (<Pg) leads to a
greater net rate of adsorption for a given adsorption capacity and better
cycle efficiency. Although a simple model was used to demonstrate this the
conclusion is valid for more realistic models of cycles. This has been shown
to be true using an experimental rapid pressure swing adsorption apparatus
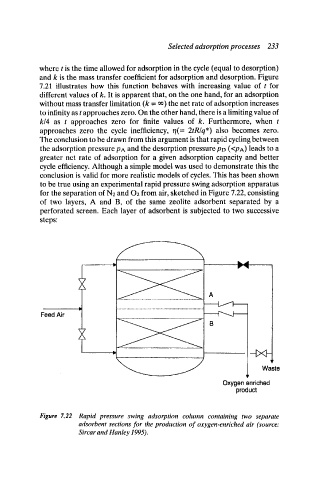
for the separation of N2 and O2 from air, sketched in Figure 7.22, consisting
of two layers, A and B, of the same zeolite adsorbent separated by a
perforated screen. Each layer of adsorbent is subjected to two successive
steps:
A
I
Feed Air
8
1
Waste
Oxygen enriched
product
Figure 7.22 Rapid pressure swing adsorption column containing two separate
adsorbent sections for the production of oxygen-enriched air (source:
Sircar and Hanley 1995).