Page 43 - Adsorption Technology & Design, Elsevier (1998)
P. 43
40 Fundamentals of adsorption equilibria
150
m
i 125
o'J
r
7
323 K
"5 100
o
G)
0
E
~: 75
0
v
~ 50
25
I I I I
0 100 200 300 400
P(Torr)
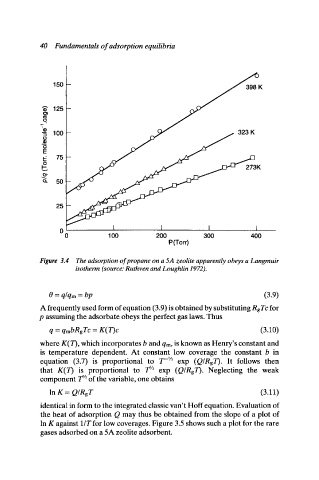
Figure 3.4 The adsorption of propane on a 5A zeolite apparently obeys a Langmuir
isotherm (source: Ruthven and Loughlin 1972).
O = q/qm = bp (3.9)
A frequently used form of equation (3.9) is obtained by substituting RgTc for
p assuming the adsorbate obeys the perfect gas laws. Thus
q = qmbRgTc = K(T)c (3.10)
where K(T), which incorporates b and qm, is known as Henry's constant and
is temperature dependent. At constant low coverage the constant b in
equation (3.7) is proportional to T -'/~ exp (Q/RgT). It follows then
that K(T) is proportional to 7 ~/~ exp (Q/RgT). Neglecting the weak
component/~/2 of the variable, one obtains
In K = Q/RgT (3.11)
identical in form to the integrated classic van't Hoff equation. Evaluation of
the heat of adsorption Q may thus be obtained from the slope of a plot of
In K against 1/T for low coverages. Figure 3.5 shows such a plot for the rare
gases adsorbed on a 5A zeolite adsorbent.