Page 145 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 145
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 141
3.6 T w ed Beds o-Phase Fix 141
a filtering catalyst make the close control of temperature and the maintaining of optimum
temperature conditions rather impossible.
3.6.2 Modeling of fixed beds
wing,
In the follo the one-dimensional model will be presented. The basic ideal models
assume that concentration and temperature gradients occur in the axial direction (Froment
and Bischoff, 1990). The model for a fed-bed reactor consists of three equations, which ix
will be presented in the following sections and are
• material balance equation
• energy balance equation
• pressure drop equation
Material balance equation
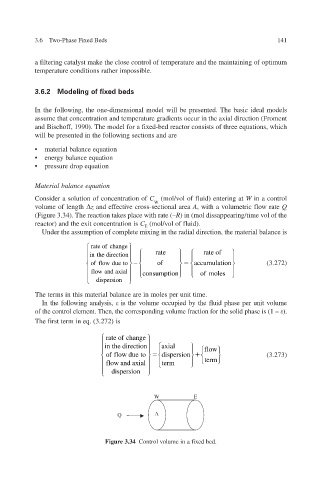
Consider a solution of concentration of C (mol/vol of fluid) entering at W in a control
W
volume of length z and efe cross-sectional area v fecti A , with a volumetric flow rate Q
(Figure 3.34). The reaction takes place with rate (– R ) in (mol dissappearing/time v ol of the
reactor) and the exit concentration is C E (mol/vol of fluid).
Under the assumption of complete mixing in the radial direction, the material balance is
rate of change
in the direction rate rate of
of flow due to of accumulation (3.272)
a
flow and axial
dispersionn consumption of moles
The terms in this material balance are in moles per unit time.
In the follo wing analysis, is the volume occupied by the fluid phase per unit v olume
of the control element. Then, the corresponding volume fraction for the solid phase is (1 – ).
The first term in eq. (3.272) is
rate of c ha nge
in the dir e tion c axial
flow
of flow due to dispersion (3.273)
flow a a nd xia l term term
dispersion n
W E
Q A
Figure 3.34 Control volume in a f ed bed. ix