Page 360 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 360
Else_AIEC-INGLE_cH005.qxd 7/12/2006 10:03 AM Page 357
5.2 Basic Principles of Catalysis 357
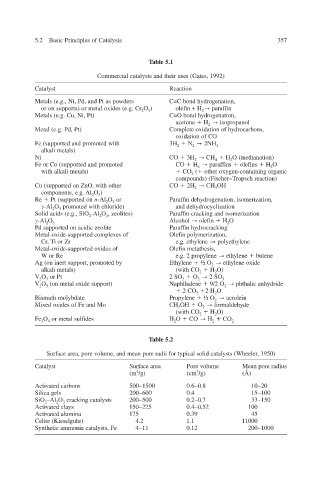
Table 5.1
Commercial catalysts and their uses (Gates, 1992)
Catalyst Reaction
Metals (e.g., Ni, Pd, and Pt as po wders C=C bond hydrogenation,
or on supports) or metal oxides (e.g. Cr 2 O 3 ) olefin + H 2 paraf in f
Metals (e.g. Cu, Ni, Pt) C=O bond hydrogenation,
acetone H 2 isopropanol
Metal (e.g. Pd, Pt) Complete oxidation of hydrocarbons,
oxidation of CO
Fe (supported and promoted with 3H 2 N 2 2NH 3
alkali metals)
Ni CO 3H 2 CH 4 H 2 O (methanation)
Fe or Co (supported and promoted CO H 2 paraf ins f olef ins H 2 O
with alkali metals) CO 2 ( other oxygen-containing or ganic
compounds) (Fischer–Tropsch reaction)
Cu (supported on ZnO, with other CO 2H 2 CH 3 OH
components, e.g. Al 2 O 3 )
Re Pt (supported on n -Al 2 O 3 or Paraffin dehydrogenation, isomerization,
-Al 2 O 3 promoted with chloride) and dehydroc yclization
Solid acids (e.g., SiO 2 -Al 2 O 3 , zeolites) Paraffin cracking and isomerization
-Al 2 O 3 Alcohol olef in H 2 O
Pd supported on acidic zeolite Paraffin hydrocracking
x Metal-oxide-supported complees of Olefin polymerization,
Cr, Ti or Zr e.g. ethylene polyethylene
Metal-oxide-supported oxides of Olefin metathesis,
W or Re e.g. 2 prop ylene ethylene b utene
Ag (on inert support, promoted by Ethylene ½ O 2 ethylene oxide
alkali metals) (with CO 2 H 2 O)
V 2 O 5 or Pt 2 SO 2 O 2 2 SO 3
V 2 O 5 (on metal oxide support) Naphthalene 9/2 O 2 phthalic anhydride
2 CO 2 2 H 2 O
Bismuth molybdate Propylene ½ O 2 acrolein
Mixed oxides of Fe and Mo CH 3 OH O 2 formaldehyde
(with CO 2 H 2 O)
Fe 3 O 4 or metal sulf ides H 2 O CO H 2 CO 2
Table 5.2
olume,
Surface area, pore v and mean pore radii for typical solid catalysts (Wheeler 1950) ,
Catalyst Surface area olume Pore v Mean pore radius
(m 2 /g) (cm 3 /g) (Å)
Activated carbons 500 ∼ 1500 0.6 ∼ 0.8 10 ∼ 20
Silica gels 200 ∼ 600 0.4 15 ∼ 100
SiO 3 –Al 2 O 3 cracking catalysts 200 ∼ 500 0.2 ∼ 0.7 33 ∼ 150
Activated clays 150 ∼ 225 0.4 ∼ 0.52 100
Activated alumina 175 0.39 45
Celite (Kieselguhr) 4.2 1.1 11000
Synthetic ammonia catalysts, Fe 4 ∼ 11 0.12 200 ∼ 1000