Page 1032 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1032
1016
15
CHAPTER 11
Free Radical Reactions Bu t
C H cyclo
6 11
10 i Pe neo
ln[k β (R)/k β (Me)] 5 Pr Pr n Et
Me
0
40 45 50 55
ΔH(R )-ΔH(RH)/Kcal mol –1
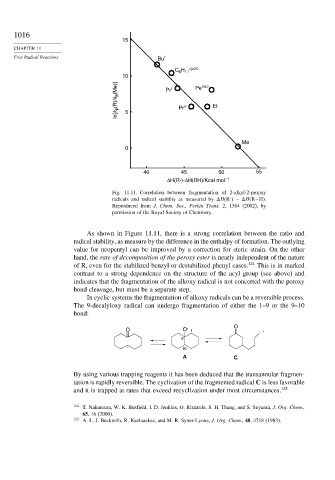
Fig. 11.11. Correlation between fragmentation of 2-alkyl-2-propxy
.
radicals and radical stability as measured by H R
– H(R−H).
Reproduced from J. Chem. Soc., Perkin Trans. 2, 1364 (2002), by
permission of the Royal Society of Chemistry.
As shown in Figure 11.11, there is a strong correlation between the ratio and
radical stability, as measure by the difference in the enthalpy of formation. The outlying
value for neopentyl can be improved by a correction for steric strain. On the other
hand, the rate of decomposition of the peroxy ester is nearly independent of the nature
of R, even for the stabilized benzyl or destabilized phenyl cases. 124 This is in marked
contrast to a strong dependence on the structure of the acyl group (see above) and
indicates that the fragmentation of the alkoxy radical is not concerted with the peroxy
bond cleavage, but must be a separate step.
In cyclic systems the fragmentation of alkoxy radicals can be a reversible process.
The 9-decalyloxy radical can undergo fragmentation of either the 1–9 or the 9–10
bond:
O
O O 1
9
10
A C
By using various trapping reagents it has been deduced that the transannular fragmen-
tation is rapidly reversible. The cyclization of the fragmented radical C is less favorable
and it is trapped at rates that exceed recyclization under most circumstances. 125
124 T. Nakamura, W. K. Busfield, I. D. Jenkins, O. Rizzardo, S. H. Thang, and S. Suyama, J. Org. Chem.,
65, 16 (2000).
125
A. L. J. Beckwith, R. Kazlauskas, and M. R. Syner-Lyons, J. Org. Chem., 48, 4718 (1983).