Page 250 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 250
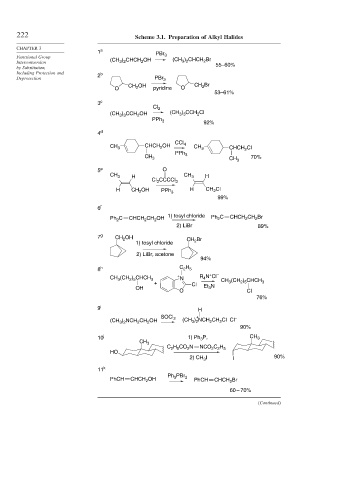
222 Scheme 3.1. Preparation of Alkyl Halides
CHAPTER 3
1 a PBr
Functional Group (CH ) CHCH OH 3 (CH ) CHCH Br
Interconversion 3 2 2 3 2 2 55–60%
by Substitution,
Including Protection and 2 b
Deprotection PBr 3
OH CH 2 Br
O CH 2 pyridine O
53–61%
3 c
Cl 2
(CH 3 ) 3 CCH 2 OH (CH ) CCH Cl
3 3
2
PPh 3
92%
4 d
CHCH OH CCl 4
CH 3 2 CH 3 CHCH 2 Cl
PPh
CH 3 3 CH 3 70%
5 e O
CH 3 H CH 3 H
Cl CCCCl 3
3
H CH OH PPh 3 H CH Cl
2
2
99%
6 f
1) tosyl chloride
Ph C CHCH CH OH Ph 2 C CHCH CH Br
2
2
2
2
2
2) LiBr 89%
7 g CH OH CH Br
2
1) tosyl chloride 2
2) LiBr, acetone
94%
8 h C H
2 5
+
CH (CH ) CHCH 3 + N R N Cl – CH (CH ) CHCH
4
2 5
3
+ Cl 3 2 5 3
OH Et 3 N
O Cl
76%
9 i H
SOCl 2 + –
(CH ) NCH CH OH (CH ) NCH CH Cl Cl
3 2
2
2
2
2
3 2
90%
10 j 1) Ph 3 P, CH 3
CH 3
C H CO N NCO C H
2 2 5
2
2 5
HO
2) CH I I 90%
3
11 k
PBr
Ph 3
PhCH CHCH OH 2 PhCH CHCH Br
2
2
60 – 70%
(Continued)