Page 103 - Advanced Thermodynamics for Engineers, Second Edition
P. 103
4.9 EXERGY 89
The change of exergy of the universe is given by
DB univ ¼ DB h þ DB c ¼ðU 2h U 1h Þ T 0 ðS 2h S 1h ÞþðU 2c U 1c Þ T 0 ðS 2c S 1c Þ
(4.58)
¼ T 0 ðS 2h S 1h þ S 2c S 1c Þ
The value of DB univ < 0 because the entropy change of the cold steam is greater than that of the hot
stream. Hence, in this irreversible heat transfer device there has been a loss of exergy in the universe
and energy has been degraded. This means that the maximum useful work available from the cold
stream is less than that available from the hot stream, even though the energy contents of the two
streams is the same.
This result has a further significance, which is that, to obtain the maximum transference of exergy,
it is important to reduce the value of T 0 (S 1 S 2 ). If the total amount of energy being transferred is kept
constant then the loss of exergy can be minimised by increasing the temperature at which the heat is
transferred. This is effectively shown in Fig. 4.11, where the high-temperature stream can be equiv-
alent to a high-temperature source of heat, while the low-temperature stream is equivalent to a low-
temperature source of heat. The quality of the heat (energy) in the high-temperature source is better
than that in the low-temperature one.
4.9.1.2 Irreversible heat transfer
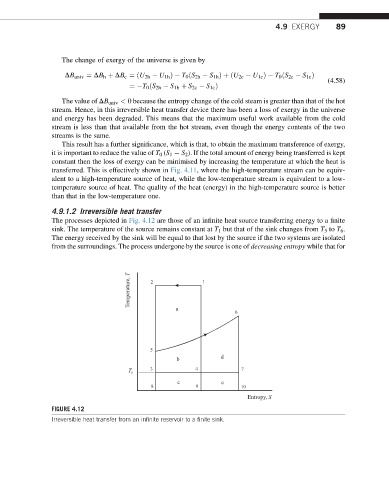
The processes depicted in Fig. 4.12 are those of an infinite heat source transferring energy to a finite
sink. The temperature of the source remains constant at T 1 but that of the sink changes from T 5 to T 6 .
The energy received by the sink will be equal to that lost by the source if the two systems are isolated
from the surroundings. The process undergone by the source is one of decreasing entropy while that for
Temperature, T 2 1
a
6
5
b d
T 3 4 7
c e
8 9 10
Entropy, S
FIGURE 4.12
Irreversible heat transfer from an infinite reservoir to a finite sink.