Page 265 - Advanced Thermodynamics for Engineers, Second Edition
P. 265
254 CHAPTER 12 CHEMICAL EQUILIBRIUM AND DISSOCIATION
If the fuel was benzene (C 6 H 6 ), then Eqn (12.35) would become
C 6 H 6 þ 7:5ðO 2 þ 3:76N 2 Þ
/6ð1 a 1 ÞCO 2 þ 3ð1 a 2 ÞH 2 O þ 6a 1 CO þ 3a 2 H 2 þð3a 1 þ 1:5a 2 ÞO 2 þ 28:2N 2
(12.36)
If the mixture were not stoichiometric, then Eqn (12.33) would be modified to take account of the
air–fuel ratio and Eqns (12.35) and (12.36) would also be modified. These equations are returned to in
the later examples. In Eqn (12.36), the potential combination of nitrogen and oxygen has been
neglected. In many combustion processes the oxygen and nitrogen join together at high temperatures
to form compounds of these elements; one of these compounds is nitric oxide (NO) and the equations
can be extended to include this reaction. This will be introduced later.
12.4.4 GENERAL OBSERVATION
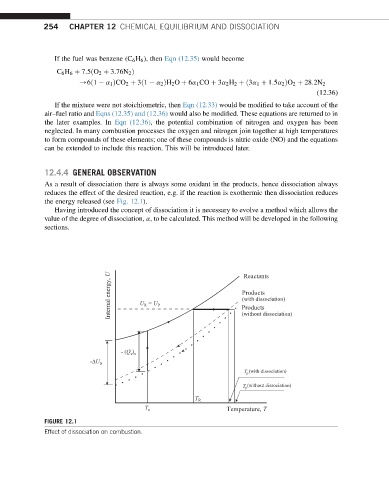
As a result of dissociation there is always some oxidant in the products, hence dissociation always
reduces the effect of the desired reaction, e.g. if the reaction is exothermic then dissociation reduces
the energy released (see Fig. 12.1).
Having introduced the concept of dissociation it is necessary to evolve a method which allows the
value of the degree of dissociation, a, to be calculated. This method will be developed in the following
sections.
Internal energy, U U = U P Products
Reactants
(with dissociation)
R
Products
(without dissociation)
- (Q )
v s
-ΔU 0
(with dissociation)
T p
(without dissociation)
T p
T R
T s Temperature, T
FIGURE 12.1
Effect of dissociation on combustion.