Page 39 - Advanced Thermodynamics for Engineers, Second Edition
P. 39
2.10 HELMHOLTZ ENERGY (HELMHOLTZ FUNCTION) 23
In the previous section the criteria for equilibrium were discussed and these were derived in terms
of DS) E . The variation of entropy is not always easy to visualise, and it would be more useful if the
criteria could be derived in a more tangible form related to other properties of the system under
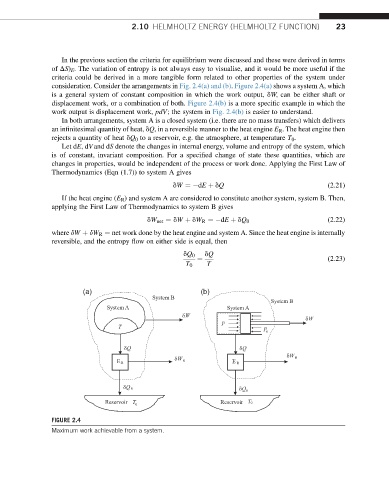
consideration. Consider the arrangements in Fig. 2.4(a) and (b). Figure 2.4(a) shows a system A, which
is a general system of constant composition in which the work output, dW, can be either shaft or
displacement work, or a combination of both. Figure 2.4(b) is a more specific example in which the
work output is displacement work, pdV; the system in Fig. 2.4(b) is easier to understand.
In both arrangements, system A is a closed system (i.e. there are no mass transfers) which delivers
an infinitesimal quantity of heat, dQ, in a reversible manner to the heat engine E R . The heat engine then
rejects a quantity of heat dQ 0 to a reservoir, e.g. the atmosphere, at temperature T 0 .
Let dE,dVand dS denote the changes in internal energy, volume and entropy of the system, which
is of constant, invariant composition. For a specified change of state these quantities, which are
changes in properties, would be independent of the process or work done. Applying the First Law of
Thermodynamics (Eqn (1.7)) to system A gives
dW ¼ dE þ dQ (2.21)
If the heat engine (E R ) and system A are considered to constitute another system, system B. Then,
applying the First Law of Thermodynamics to system B gives
dW net ¼ dW þ dW R ¼ dE þ dQ 0 (2.22)
where dW þ dW R ¼ net work done by the heat engine and system A. Since the heat engine is internally
reversible, and the entropy flow on either side is equal, then
dQ 0 dQ
¼ (2.23)
T 0 T
(a) (b)
System B
System B
System A System A
δW δW
p
T p
0
δQ δQ
δW R
δW R
E R E R
δQ 0
δQ 0
Reservoir T Reservoir
0 T 0
FIGURE 2.4
Maximum work achievable from a system.