Page 89 - Advanced Thermodynamics for Engineers, Second Edition
P. 89
4.7 AVAILABILITY BALANCE FOR A CLOSED SYSTEM 75
Δ S H
T H
T H A
Q H Temperature, T
W
E T 0
- Q 0 I
Δ S 0 Entropy, S
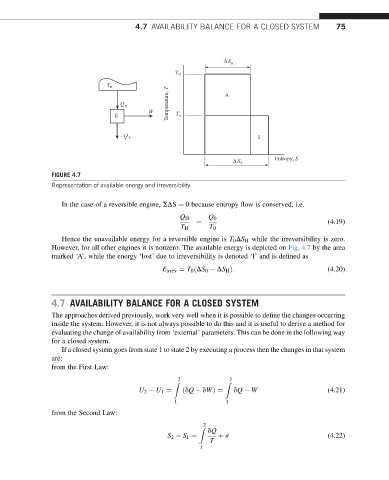
FIGURE 4.7
Representation of available energy and irreversibility.
In the case of a reversible engine, SDS ¼ 0 because entropy flow is conserved, i.e.
Q H Q 0
¼ (4.19)
T H T 0
Hence the unavailable energy for a reversible engine is T 0 DS H while the irreversibility is zero.
However, for all other engines it is nonzero. The available energy is depicted on Fig. 4.7 by the area
marked ‘A’, while the energy ‘lost’ due to irreversibility is denoted ‘I’ and is defined as
E irrev ¼ T 0 ðDS 0 DS H Þ: (4.20)
4.7 AVAILABILITY BALANCE FOR A CLOSED SYSTEM
The approaches derived previously, work very well when it is possible to define the changes occurring
inside the system. However, it is not always possible to do this and it is useful to derive a method for
evaluating the change of availability from ‘external’ parameters. This can be done in the following way
for a closed system.
If a closed system goes from state 1 to state 2 by executing a process then the changes in that system
are:
from the First Law:
Z 2 Z 2
U 2 U 1 ¼ ðdQ dWÞ¼ dQ W (4.21)
1 1
from the Second Law:
Z 2
dQ
S 2 S 1 ¼ þ s (4.22)
T
1