Page 96 - Advanced Thermodynamics for Engineers, Second Edition
P. 96
82 CHAPTER 4 AVAILABILITY AND EXERGY
1.2
Availability received
during compression Availability destroyed
during combustion
1.0
Non-dimensional availability , a/q* 0.8 Availability in charge Top dead centre Availability delivered as
work during expansion
0.6
0.4
0.2
0
-180 -135 -90 -45 0 45 90 135 180
Crankangle / (deg)
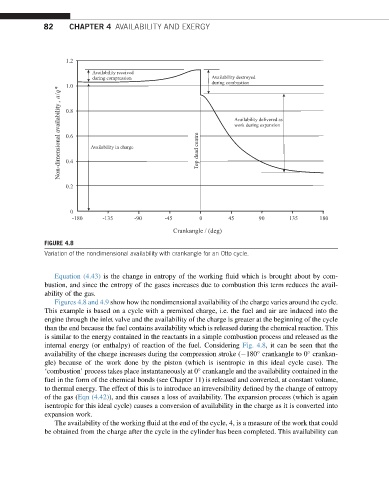
FIGURE 4.8
Variation of the nondimensional availability with crankangle for an Otto cycle.
Equation (4.43) is the change in entropy of the working fluid which is brought about by com-
bustion, and since the entropy of the gases increases due to combustion this term reduces the avail-
ability of the gas.
Figures 4.8 and 4.9 show how the nondimensional availability of the charge varies around the cycle.
This example is based on a cycle with a premixed charge, i.e. the fuel and air are induced into the
engine through the inlet valve and the availability of the charge is greater at the beginning of the cycle
than the end because the fuel contains availability which is released during the chemical reaction. This
is similar to the energy contained in the reactants in a simple combustion process and released as the
internal energy (or enthalpy) of reaction of the fuel. Considering Fig. 4.8, it can be seen that the
availability of the charge increases during the compression stroke ( 180 crankangle to 0 crankan-
gle) because of the work done by the piston (which is isentropic in this ideal cycle case). The
‘combustion’ process takes place instantaneously at 0 crankangle and the availability contained in the
fuel in the form of the chemical bonds (see Chapter 11) is released and converted, at constant volume,
to thermal energy. The effect of this is to introduce an irreversibility defined by the change of entropy
of the gas (Eqn (4.42)), and this causes a loss of availability. The expansion process (which is again
isentropic for this ideal cycle) causes a conversion of availability in the charge as it is converted into
expansion work.
The availability of the working fluid at the end of the cycle, 4, is a measure of the work that could
be obtained from the charge after the cycle in the cylinder has been completed. This availability can