Page 307 - Advanced thermodynamics for engineers
P. 307
CHAPTER
13
EFFECT OF DISSOCIATION ON
COMBUSTION PARAMETERS
Dissociation is an equilibrium process by which the products of combustion achieve the minimum
Gibbs energy for the mixture (see Chapter 12). The effect is to cause the products that would be
obtained from complete combustion to break down partially into the original reactants, and other
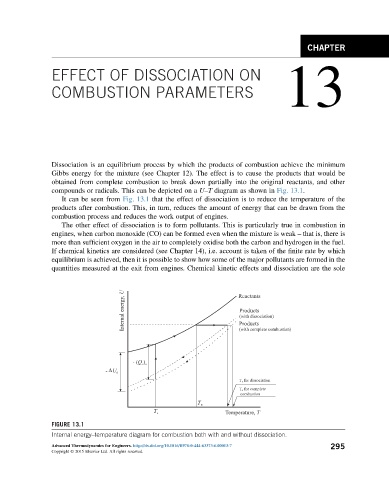
compounds or radicals. This can be depicted on a U–T diagram as shown in Fig. 13.1.
It can be seen from Fig. 13.1 that the effect of dissociation is to reduce the temperature of the
products after combustion. This, in turn, reduces the amount of energy that can be drawn from the
combustion process and reduces the work output of engines.
The other effect of dissociation is to form pollutants. This is particularly true in combustion in
engines, when carbon monoxide (CO) can be formed even when the mixture is weak – that is, there is
more than sufficient oxygen in the air to completely oxidise both the carbon and hydrogen in the fuel.
If chemical kinetics are considered (see Chapter 14), i.e. account is taken of the finite rate by which
equilibrium is achieved, then it is possible to show how some of the major pollutants are formed in the
quantities measured at the exit from engines. Chemical kinetic effects and dissociation are the sole
Internal energy, U Reactants
Products
(with dissociation)
Products
(with complete combustion)
- (Q )
Δ
- U
T for dissociation
T for complete
combustion
T R
T s Temperature, T
FIGURE 13.1
Internal energy–temperature diagram for combustion both with and without dissociation.
Advanced Thermodynamics for Engineers. http://dx.doi.org/10.1016/B978-0-444-63373-6.00013-7 295
Copyright © 2015 Elsevier Ltd. All rights reserved.