Page 414 - Advanced Mine Ventilation
P. 414
Gas and Dust Explosions 383
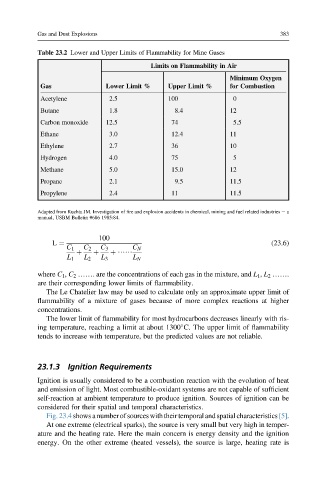
Table 23.2 Lower and Upper Limits of Flammability for Mine Gases
Limits on Flammability in Air
Minimum Oxygen
Gas Lower Limit % Upper Limit % for Combustion
Acetylene 2.5 100 0
Butane 1.8 8.4 12
Carbon monoxide 12.5 74 5.5
Ethane 3.0 12.4 11
Ethylene 2.7 36 10
Hydrogen 4.0 75 5
Methane 5.0 15.0 12
Propane 2.1 9.5 11.5
Propylene 2.4 11 11.5
Adapted from Kuchta JM. Investigation of fire and explosion accidents in chemical, mining and fuel related industries e a
manual, USBM Bulletin #686 1985:84.
100
L ¼ (23.6)
C 1 C 2 C 3 C N
þ þ þ //
L 1 L 2 L 3 L N
where C 1 , C 2 ... are the concentrations of each gas in the mixture, and L 1 , L 2 ...
are their corresponding lower limits of flammability.
The Le Chatelier law may be used to calculate only an approximate upper limit of
flammability of a mixture of gases because of more complex reactions at higher
concentrations.
The lower limit of flammability for most hydrocarbons decreases linearly with ris-
ing temperature, reaching a limit at about 1300 C. The upper limit of flammability
tends to increase with temperature, but the predicted values are not reliable.
23.1.3 Ignition Requirements
Ignition is usually considered to be a combustion reaction with the evolution of heat
and emission of light. Most combustible-oxidant systems are not capable of sufficient
self-reaction at ambient temperature to produce ignition. Sources of ignition can be
considered for their spatial and temporal characteristics.
Fig. 23.4 shows anumberof sources with theirtemporal andspatialcharacteristics [5].
At one extreme (electrical sparks), the source is very small but very high in temper-
ature and the heating rate. Here the main concern is energy density and the ignition
energy. On the other extreme (heated vessels), the source is large, heating rate is