Page 225 - Advances in Textile Biotechnology
P. 225
206 Advances in textile biotechnology
OH OH O
(a)
OH O
Tyrosinase Tyrosinase
1 1
O 2 + O 2 + H 2O
2 2
Tyrosine Dopa Dopaquinone
(b)
O O
HO OH [1] [2]
O N
or
HN
2
2
HO OH H C H C
Phenol coupling Reaction with nucleophiles
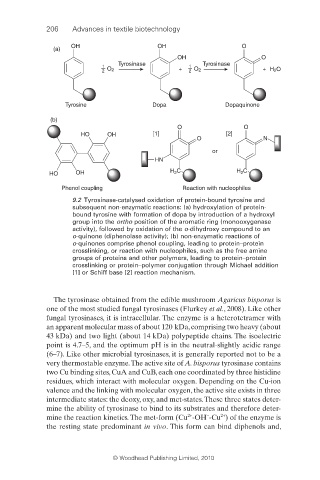
9.2 Tyrosinase-catalysed oxidation of protein-bound tyrosine and
subsequent non-enzymatic reactions: (a) hydroxylation of protein-
bound tyrosine with formation of dopa by introduction of a hydroxyl
group into the ortho position of the aromatic ring (monooxygenase
activity), followed by oxidation of the o-dihydroxy compound to an
o-quinone (diphenolase activity); (b) non-enzymatic reactions of
o-quinones comprise phenol coupling, leading to protein–protein
crosslinking, or reaction with nucleophiles, such as the free amine
groups of proteins and other polymers, leading to protein–protein
crosslinking or protein–polymer conjugation through Michael addition
[1] or Schiff base [2] reaction mechanism.
The tyrosinase obtained from the edible mushroom Agaricus bisporus is
one of the most studied fungal tyrosinases (Flurkey et al., 2008). Like other
fungal tyrosinases, it is intracellular. The enzyme is a heterotetramer with
an apparent molecular mass of about 120 kDa, comprising two heavy (about
43 kDa) and two light (about 14 kDa) polypeptide chains. The isoelectric
point is 4.7–5, and the optimum pH is in the neutral-slightly acidic range
(6–7). Like other microbial tyrosinases, it is generally reported not to be a
very thermostable enzyme. The active site of A. bisporus tyrosinase contains
two Cu binding sites, CuA and CuB, each one coordinated by three histidine
residues, which interact with molecular oxygen. Depending on the Cu-ion
valence and the linking with molecular oxygen, the active site exists in three
intermediate states: the deoxy, oxy, and met-states. These three states deter-
mine the ability of tyrosinase to bind to its substrates and therefore deter-
−
2+
2+
mine the reaction kinetics. The met-form (Cu -OH -Cu ) of the enzyme is
the resting state predominant in vivo. This form can bind diphenols and,
© Woodhead Publishing Limited, 2010