Page 98 - Aeronautical Engineer Data Book
P. 98
Basic fluid mechanics 77
well above their liquefication temperature will
approximate to it and so they can be considered
as a perfect gas.
5.1.3 Changes of state
When a perfect gas changes state its behaviour
approximates to:
n
pv = constant
where n is known as the polytropic exponent.
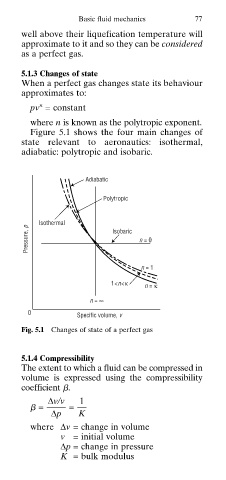
Figure 5.1 shows the four main changes of
state relevant to aeronautics: isothermal,
adiabatic: polytropic and isobaric.
Adiabatic
Polytropic
Pressure, p Isothermal Isobaric n = 0
n = 1
1<n<κ n = κ
n = ∞
0 Specific volume, v
Fig. 5.1 Changes of state of a perfect gas
5.1.4 Compressibility
The extent to which a fluid can be compressed in
volume is expressed using the compressibility
coefficient .
∆v/v 1
=
=
∆p K
where ∆v = change in volume
v = initial volume
∆p = change in pressure
K = bulk modulus