Page 110 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 110
84 3 Basics of Gas Combustion
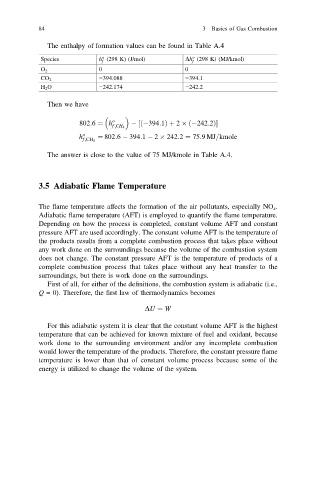
The enthalpy of formation values can be found in Table A.4
o
o
Species h (298 K) (J/mol) Dh (298 K) (MJ/kmol)
f
f
O 2 0 0
−394.088 −394.1
CO 2
H 2 O −242.174 −242.2
Then we have
802:6 ¼ h o 394:1Þ þ 2 242:2Þ
½
ð
ð
f ;CH 4
h o ¼ 802:6 394:1 2 242:2 ¼ 75:9MJ=kmole
f ;CH 4
The answer is close to the value of 75 MJ/kmole in Table A.4.
3.5 Adiabatic Flame Temperature
The flame temperature affects the formation of the air pollutants, especially NO x .
Adiabatic flame temperature (AFT) is employed to quantify the flame temperature.
Depending on how the process is completed, constant volume AFT and constant
pressure AFT are used accordingly. The constant volume AFT is the temperature of
the products results from a complete combustion process that takes place without
any work done on the surroundings because the volume of the combustion system
does not change. The constant pressure AFT is the temperature of products of a
complete combustion process that takes place without any heat transfer to the
surroundings, but there is work done on the surroundings.
First of all, for either of the definitions, the combustion system is adiabatic (i.e.,
Q = 0). Therefore, the first law of thermodynamics becomes
DU ¼ W
For this adiabatic system it is clear that the constant volume AFT is the highest
temperature that can be achieved for known mixture of fuel and oxidant, because
work done to the surrounding environment and/or any incomplete combustion
would lower the temperature of the products. Therefore, the constant pressure flame
temperature is lower than that of constant volume process because some of the
energy is utilized to change the volume of the system.