Page 158 - Algae
P. 158
Photosynthesis 141
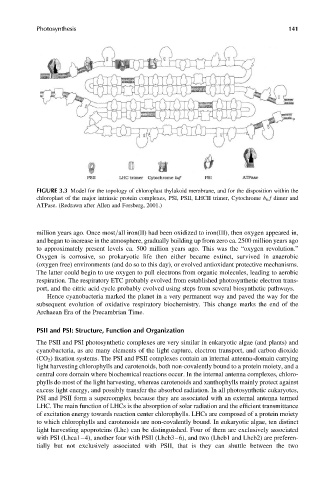
FIGURE 3.3 Model for the topology of chloroplast thylakoid membrane, and for the disposition within the
chloroplast of the major intrinsic protein complexes, PSI, PSII, LHCII trimer, Cytochrome b 6 f dimer and
ATPase. (Redrawn after Allen and Forsberg, 2001.)
million years ago. Once most/all iron(II) had been oxidized to iron(III), then oxygen appeared in,
and began to increase in the atmosphere, gradually building up from zero ca. 2500 million years ago
to approximately present levels ca. 500 million years ago. This was the “oxygen revolution.”
Oxygen is corrosive, so prokaryotic life then either became extinct, survived in anaerobic
(oxygen free) environments (and do so to this day), or evolved antioxidant protective mechanisms.
The latter could begin to use oxygen to pull electrons from organic molecules, leading to aerobic
respiration. The respiratory ETC probably evolved from established photosynthetic electron trans-
port, and the citric acid cycle probably evolved using steps from several biosynthetic pathways.
Hence cyanobacteria marked the planet in a very permanent way and paved the way for the
subsequent evolution of oxidative respiratory biochemistry. This change marks the end of the
Archaean Era of the Precambrian Time.
PSII and PSI: Structure, Function and Organization
The PSII and PSI photosynthetic complexes are very similar in eukaryotic algae (and plants) and
cyanobacteria, as are many elements of the light capture, electron transport, and carbon dioxide
(CO 2 ) fixation systems. The PSI and PSII complexes contain an internal antenna-domain carrying
light harvesting chlorophylls and carotenoids, both non-covalently bound to a protein moiety, and a
central core domain where biochemical reactions occur. In the internal antenna complexes, chloro-
phylls do most of the light harvesting, whereas carotenoids and xanthophylls mainly protect against
excess light energy, and possibly transfer the absorbed radiation. In all photosynthetic eukaryotes,
PSI and PSII form a supercomplex because they are associated with an external antenna termed
LHC. The main function of LHCs is the absorption of solar radiation and the efficient transmittance
of excitation energy towards reaction center chlorophylls. LHCs are composed of a protein moiety
to which chlorophylls and carotenoids are non-covalently bound. In eukaryotic algae, ten distinct
light harvesting apoproteins (Lhc) can be distinguished. Four of them are exclusively associated
with PSI (Lhca1–4), another four with PSII (Lhcb3–6), and two (Lhcb1 and Lhcb2) are preferen-
tially but not exclusively associated with PSII, that is they can shuttle between the two