Page 168 - Algae
P. 168
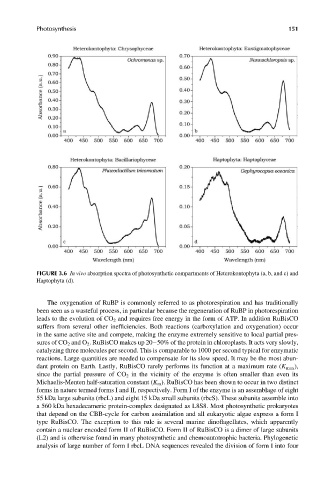
Photosynthesis 151
FIGURE 3.6 In vivo absorption spectra of photosynthetic compartments of Heterokontophyta (a, b, and c) and
Haptophyta (d).
The oxygenation of RuBP is commonly referred to as photorespiration and has traditionally
been seen as a wasteful process, in particular because the regeneration of RuBP in photorespiration
leads to the evolution of CO 2 and requires free energy in the form of ATP. In addition RuBisCO
suffers from several other inefficiencies. Both reactions (carboxylation and oxygenation) occur
in the same active site and compete, making the enzyme extremely sensitive to local partial pres-
sures of CO 2 and O 2 . RuBisCO makes up 20–50% of the protein in chloroplasts. It acts very slowly,
catalyzing three molecules per second. This is comparable to 1000 per second typical for enzymatic
reactions. Large quantities are needed to compensate for its slow speed. It may be the most abun-
dant protein on Earth. Lastly, RuBisCO rarely performs its function at a maximum rate (K max ),
since the partial pressure of CO 2 in the vicinity of the enzyme is often smaller than even its
Michaelis-Menten half-saturation constant (K m ). RuBisCO has been shown to occur in two distinct
forms in nature termed forms I and II, respectively. Form I of the enzyme is an assemblage of eight
55 kDa large subunits (rbcL) and eight 15 kDa small subunits (rbcS). These subunits assemble into
a 560 kDa hexadecameric protein-complex designated as L8S8. Most photosynthetic prokaryotes
that depend on the CBB-cycle for carbon assimilation and all eukaryotic algae express a form I
type RuBisCO. The exception to this rule is several marine dinoflagellates, which apparently
contain a nuclear encoded form II of RuBisCO. Form II of RuBisCO is a dimer of large subunits
(L2) and is otherwise found in many photosynthetic and chemoautotrophic bacteria. Phylogenetic
analysis of large number of form I rbcL DNA sequences revealed the division of form I into four