Page 88 - An Introduction to Analytical Atomic Spectrometry - L. Ebdon
P. 88
Page 70
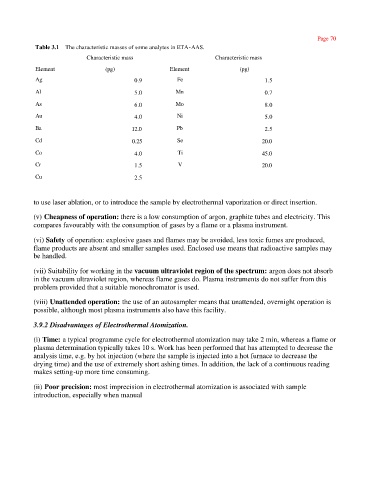
Table 3.1 The characteristic masses of some analytes in ETA-AAS.
Characteristic mass Characteristic mass
Element (pg) Element (pg)
Ag 0.9 Fe 1.5
Al 5.0 Mn 0.7
As 6.0 Mo 8.0
Au 4.0 Ni 5.0
Ba 12.0 Pb 2.5
Cd 0.25 Se 20.0
Co 4.0 Ti 45.0
Cr 1.5 V 20.0
Cu 2.5
to use laser ablation, or to introduce the sample by electrothermal vaporization or direct insertion.
(v) Cheapness of operation: there is a low consumption of argon, graphite tubes and electricity. This
compares favourably with the consumption of gases by a flame or a plasma instrument.
(vi) Safety of operation: explosive gases and flames may be avoided, less toxic fumes are produced,
flame products are absent and smaller samples used. Enclosed use means that radioactive samples may
be handled.
(vii) Suitability for working in the vacuum ultraviolet region of the spectrum: argon does not absorb
in the vacuum ultraviolet region, whereas flame gases do. Plasma instruments do not suffer from this
problem provided that a suitable monochromator is used.
(viii) Unattended operation: the use of an autosampler means that unattended, overnight operation is
possible, although most plasma instruments also have this facility.
3.9.2 Disadvantages of Electrothermal Atomization.
(i) Time: a typical programme cycle for electrothermal atomization may take 2 min, whereas a flame or
plasma determination typically takes 10 s. Work has been performed that has attempted to decrease the
analysis time, e.g. by hot injection (where the sample is injected into a hot furnace to decrease the
drying time) and the use of extremely short ashing times. In addition, the lack of a continuous reading
makes setting-up more time consuming.
(ii) Poor precision: most imprecision in electrothermal atomization is associated with sample
introduction, especially when manual