Page 194 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 194
Risk to the patient—Quantifying assurance of sterility 175
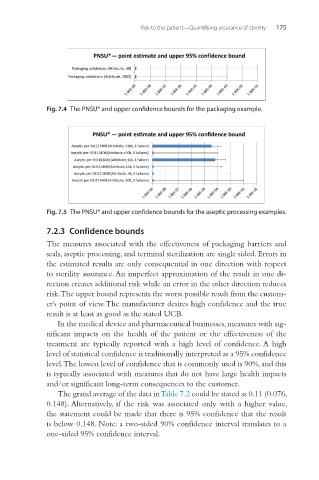
Fig. 7.4 The PNSU* and upper confidence bounds for the packaging example.
Fig. 7.5 The PNSU* and upper confidence bounds for the aseptic processing examples.
7.2.3 Confidence bounds
The measures associated with the effectiveness of packaging barriers and
seals, aseptic processing, and terminal sterilization are single sided. Errors in
the estimated results are only consequential in one direction with respect
to sterility assurance. An imperfect approximation of the result in one di-
rection creates additional risk while an error in the other direction reduces
risk. The upper bound represents the worst possible result from the custom-
er’s point of view. The manufacturer desires high confidence and the true
result is at least as good as the stated UCB.
In the medical device and pharmaceutical businesses, measures with sig-
nificant impacts on the health of the patient or the effectiveness of the
treatment are typically reported with a high level of confidence. A high
level of statistical confidence is traditionally interpreted as a 95% confidence
level. The lowest level of confidence that is commonly used is 90%, and this
is typically associated with measures that do not have large health impacts
and/or significant long-term consequences to the customer.
The grand average of the data in Table 7.2 could be stated as 0.11 (0.076,
0.148). Alternatively, if the risk was associated only with a higher value,
the statement could be made that there is 95% confidence that the result
is below 0.148. Note: a two-sided 90% confidence interval translates to a
one-sided 95% confidence interval.