Page 82 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 82
68 Assurance of sterility for sensitive combination products and materials
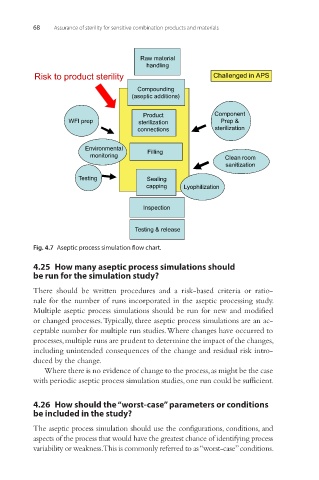
Raw material
handling
Risk to product sterility Challenged in APS
Compounding
(aseptic additions)
Product Component
WFI prep sterilization Prep &
connections sterilization
Environmental Filling
monitoring Clean room
sanitization
Testing Sealing
capping Lyophilization
Inspection
Testing & release
Fig. 4.7 Aseptic process simulation flow chart.
4.25 How many aseptic process simulations should
be run for the simulation study?
There should be written procedures and a risk-based criteria or ratio-
nale for the number of runs incorporated in the aseptic processing study.
Multiple aseptic process simulations should be run for new and modified
or changed processes. Typically, three aseptic process simulations are an ac-
ceptable number for multiple run studies. Where changes have occurred to
processes, multiple runs are prudent to determine the impact of the changes,
including unintended consequences of the change and residual risk intro-
duced by the change.
Where there is no evidence of change to the process, as might be the case
with periodic aseptic process simulation studies, one run could be sufficient.
4.26 How should the “worst-case” parameters or conditions
be included in the study?
The aseptic process simulation should use the configurations, conditions, and
aspects of the process that would have the greatest chance of identifying process
variability or weakness. This is commonly referred to as “worst-case” conditions.