Page 129 - Basic physical chemistry for the atmospheric sciences
P. 129
Oxidation-reduction reactions 1 1 '1
6.4 Half-reactions in electrochemical cells
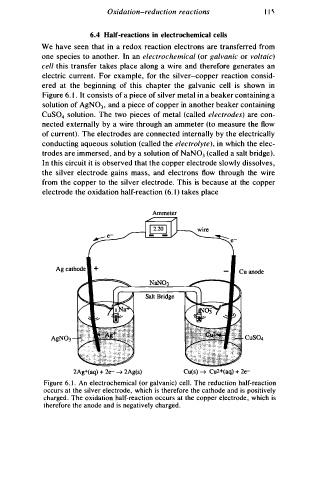
We have seen that in a redox reaction electrons are transferred from
one species to another. In an electrochemical (or galvanic or voltaic)
cell this transfer takes place along a wire and therefore generates an
electric current. For example, for the silver-copper reaction consid
ered at the beginning of this chapter the galvanic cell is shown in
Figure 6. 1 . It consists of a piece of silver metal in a beaker containing a
solution of AgN03 , and a piece of copper in another beaker containing
CuS0 solution. The two pieces of metal (called electrodes) are con
4
nected externally by a wire through an ammeter (to measure the flow
of current) . The electrodes are connected internally by the electrically
conducting aqueous solution (called the electrolyte), in which the elec
trodes are immersed , and by a solution of NaN03 (called a salt bridge).
I n this circuit it is observed that the copper electrode slowly dissolves,
s
the silver electrode gains ma s , and electrons flow through the wire
from the copper to the silver electrode. This is because at the copper
electrode the oxidation half-reaction (6. 1 ) takes place
�
e-
Ag cathode + Cu anode
Salt Bridge
2Ag + (aq) + 2e- � 2Ag(s) Cu(s) � Cu 2 +(aq) + 2e-
Figure 6. 1 . An electrochemical (or galvanic) cell. The reduction half-reaction
occurs at the silver electrode, which is therefore the cathode and is positively
charged . The oxidation half-reaction occurs at the copper electrode , which is
t h erefore the anode and is negatively charge .
d