Page 19 - Battery Reference Book
P. 19
1/4 Introduction to battery technology
or coulombs. The reaction quoted above involving the 1.1.2 Origin of electromotive force
passage into solution of one equivalent of zinc and
the deposition of one equivalent of copper is there- It is opportune at this point to consider why it comes
fore accompanied by the production of 2 F (192 988 C), about that certain reactions, when conducted in gal-
since the atomic weights of zinc and copper both con- vanic cells, give rise to an electrical current. Many
tain two equivalents. theories have been advanced to account for this phe-
nomenon. Thus, in 1801, Volta discovered that if two
insulated pieces of different metals are put in con-
1.1.1 Measurement of the electromotive force tact and then separated they acquire electric charges
The electromotive force of a cell is defined as the of opposite sign. If the metals are zinc and copper, the
potential difference between the poles when no current zinc acquires a positive charge and the copper a neg-
is flowing through the cell. When a current is flowing ative charge. There is therefore a tendency for negative
through a cell and through an external circuit, there is electricity to pass from the zinc to the copper. Volta
a fall of potential inside the cell owing to its internal believed that this tendency was mainly responsible for
resistance, and the fall of potential in the outside circuit the production of the current in the galvanic cell. The
is less than the potential difference between the poles solution served merely to separate the two metals and
at open circuit. so eliminate the contact effect at the other end.
In fact if R is the resistance of the outside cir- It soon became evident that the production of the
cuit, r the internal resistance of the cell and E its current was intimately connected with the chemical
electromotive force, the current through the circuit is: actions occurring at the electrodes, and a ‘chemical
theory’ was formulated, according to which the elec-
E
Cx- trode processes were mainly responsible for the pro-
Rfr duction of the current. Thus there arose a controversy
The potential difference between the poles is now which lasted, on and off, for a century.
only E‘ = CR, so that On the one hand the chemical theory was strength-
ened by Faraday’s discovery of the equivalence of the
E’IE = RIR + r
current produced to the amount of chemical action
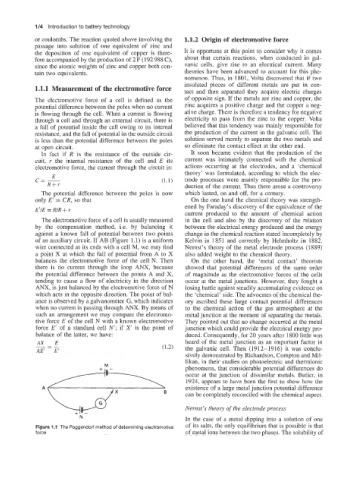
The electromotive force of a cell is usually measured in the cell and also by the discovery of the relation
by the compensation method, i.e. by balancing it between the electrical energy produced and the energy
against a known fall of potential between two points change in the chemical reaction stated incompletely by
of an auxiliary circuit. If AB (Figure 1.1) is a uniform Kelvin in 1851 and correctly by Helmholtz in 1882.
wire connected at its ends with a cell M, we may find Nernst’s theory of the metal electrode process (1889)
a point X at which the fall of potential from A to X also added weight to the chemical theory.
balances the electromotive force of the cell N. Then On the other hand, the ‘metal contact’ theorists
there is no current through the loop ANX, because showed that potential differences of the same order
the potential difference between the points A and X, of magnitude as the electromotive forces of the cells
tending to cause a flow of electricity in the direction occur at the metal junctions. However, they fought a
ANX, is just balanced by the electromotive force of N losing battle against steadily accumulating evidence on
which acts in the opposite direction. The point of bal- the ‘chemical’ side. The advocates of the chemical the-
ance is observed by a galvanometer G, which indicates ory ascribed these large contact potential differences
when no current is passing through ANX. By means of to the chemical action of the gas atmosphere at the
such an arrangement we may compare the electromo- metal junction at the moment of separating the metals.
tive force E of the cell N with a known electromotive They pointed out that no change occurred at the metal
force E’ of a standard cell N‘; if X‘ is the point of junction which could provide the electrical energy pro-
balance of the latter, we have: duced. Consequently, for 20 years after 1800 little was
A X E heard of the metal junction as an important factor in
- the galvanic cell. Then (1912-1916) it was conclu-
AX’ E‘
sively demonstrated by Richardson, Compton and Mil-
likan, in their studies on photoelectric and thermionic
M
phenomena, that considerable potential differences do
occur at the junction of dissimilar metals. Butler, in
1924, appears to have been the first to show how the
existence of a large metal junction potential difference
can be completely reconciled with the chemical aspect.
Nernst’s theory of the electrode process
N In the case of a metal dipping into a solution of one
Figure 1.1 The Poggendorf method of determining electromotive of its salts, the only equilibrium that is possible is that
force of metal ions between the two phases. The solubility of