Page 430 - Battery Reference Book
P. 430
Zinc-air primary batteries 40/3
40.1 Zinc-air primary batteries are porous to ions and are made of several layers.
These prevent the transfer of released reaction prod-
The zinc-air system, which attracted a great deal ucts and therefore ensure long-term discharge of very
of investment in the late 1960s and early 1970s to low intensity currents within short-circuits. The elec-
make a consumer product in standard cylindrical sizes, trolyte is potassium hydroxide. This cell has a spe-
suffered initially from four problems. It was difficult cific energy density of 650-800 mW h/cm3 compared
to produce air-breathing cathodes of consistent quality; with 400-520, 350-430 and 200-300, respectively,
the need to allow air into the cell led to electrolyte for mercury-zinc, silver-zinc and alkaline manganese
leakage; carbonation of the electrolyte occurred on dioxide cells. It has an operational temperature range
long-term discharge; and during intermittent discharge of -10 to +60"C and a service voltage of 1.15-1.3V.
oxygen ingress products caused wasteful corrosion of The cell has excellent shelf life because the air inlets
the active material.
Despite these initial difficulties, commercially avail- are covered by a thin foil during storage. which keeps
able D and N cells were available in the UK in the out the air needed to activate the cell. The discharge
early 1970s. The ECL D-size cell outperformed D-size performance of the Varta zinc-air cell is compared
nickel-cadmium cells under most conditions, except with that of alkaline manganese-zinc, alkaline sil-
at sub-zero temperatures, in a military 'man pack' ver-zinc and alkaline mercury-zinc cells, shown in
radio application. The cost of this zinc-air cell was Figure 40.2.
about 25% of that of an equivalent nickel-cadmium Zinc-air cells also have applications in navigation
cell and proved to be more economical. However, aids. McGraw Edison, for example, supply the
the nickel-cadmium cell had the advantage of being
rechargeable. Primary zinc-air batteries were used
extensively during the Vietnam War. The N-size cell
had been used successfully for the small pocket paging
equipment market. The demise of zinc-air D and N
cells rests on economic factors rather than on technical
grounds. The picture for zinc-air button cells is quite
different. Gould have manufactured them in the USA
for several years for applications such as hearing-aids,
watches, etc., and Gould and Berec have both been
marketing them in the UK since 1980.
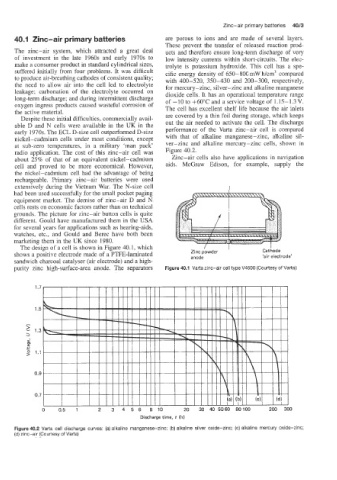
The design of a cell is shown in Figure 40.1, which ,
shows a positive electrode made of a PTFE-laminated Zinc powder I Cathode
'air electrode'
anode
sandwich charcoal catalyser (air electrode) and a high-
purity zinc high-surface-area anode. The separators Figure 40.1 Varta zinc-air cell type V4600 (Courtesy of Varta)
Figure 40.2 Varta cell discharge curves: (a) alkaline manganese-zinc; (b) alkaline silver oxide-zinc: (c) alkaline mercury oxide-zinc;
(d) zinc--air (Courtesy of Varta)