Page 580 - Battery Reference Book
P. 580
51/20 Nickel batteries
Discharge rate (PA)
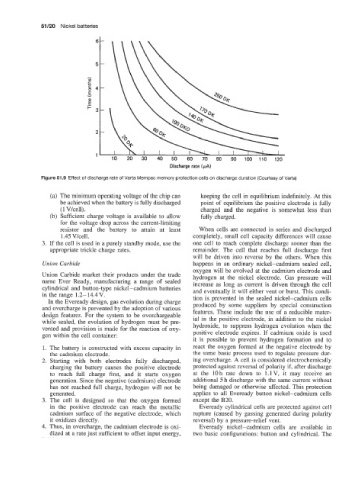
Figure 51.9 Effect of discharge rate of Varta Mempac memory protection cells on discharge duration (Courtesy of Varta)
(a) The minimum operating voltage of the chip can keeping the cell in equilibrium indefinitely. At this
be achieved when the battery is fully discharged point of equilibrium the positive electrode is fully
(1 Vkell). charged and the negative is somewhat less than
(b) Sufficient charge voltage is available to allow fully charged.
for the voltage drop across the current-limiting
resistor and the battery to attain at least When cells are connected in series and discharged
1.45 Vkell. completely, small cell capacity differences will cause
3. If the cell is used in a purely standby mode, use the one cell to reach complete discharge sooner than the
appropriate trickle charge rates. remainder. The cell that reaches full discharge first
will be driven into reverse by the others. When this
Union Carbide happens in an ordinary nickel-cadmium sealed cell,
oxygen will be evolved at the cadmium electrode and
Union Carbide market their products under the trade hydrogen at the nickel electrode. Gas pressure will
name Ever Ready, manufacturing a range of sealed increase as long as current is driven through the cell
cylindrical and button-type nickel-cadmium batteries and eventually it will either vent or burst. This condi-
in the range 1.2- 14.4 V. tion is prevented in the sealed nickel-cadmium cells
In the Eveready design, gas evolution during charge
and overcharge is prevented by the adoption of various produced by some suppliers by special construction
design features. For the system to be overchargeable features. These include the use of a reducible mater-
ial in the positive electrode, in addition to the nickel
while sealed, the evolution of hydrogen must be pre-
vented and provision is made for the reaction of oxy- hydroxide, to suppress hydrogen evolution when the
gen within the cell container: positive electrode expires. If cadmium oxide is used
it is possible to prevent hydrogen formation and to
1. The battery is constructed with excess capacity in react the oxygen formed at the negative electrode by
the cadmium electrode. the same basic process used to regulate pressure dur-
2. Starting with both electrodes fully discharged, ing overcharge. A cell is considered electrochemically
charging the battery causes the positive electrode protected against reversal of polarity if, after discharge
to reach full charge first, and it starts oxygen at the 10h rate down to l.lV, it may receive an
generation. Since the negative (cadmium) electrode additional 5 h discharge with the same current without
has not reached full charge, hydrogen will not be being damaged or otherwise affected. This protection
generated. applies to all Eveready button nickel-cadmium cells
3. The cell is designed so that the oxygen formed except the B20.
in the positive electrode can reach the metallic Eveready cylindrical cells are protected against cell
cadmium surface of the negative electrode, which rupture (caused by gassing generated during polarity
it oxidizes directly. reversal) by a pressure-relief vent.
4. Thus, in overcharge, the cadmium electrode is oxi- Eveready nickel-cadmium cells are available in
dized at a rate just sufficient to offset input energy, two basic configurations: button and cylindrical. The